Stereoselective amination of racemic sec-alcohols through sequential application of laccases and transaminases†
Abstract
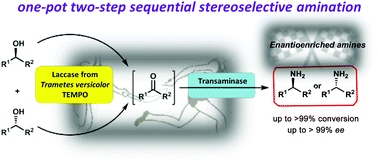
A one-pot/two-step bienzymatic asymmetric amination of secondary alcohols is disclosed. The approach is based on a sequential strategy involving the use of a laccase/TEMPO catalytic system for the oxidation of alcohols into ketone intermediates, and their following transformation into optically enriched amines by using transaminases. Individual optimizations of the oxidation and biotransamination reactions have been carried out, studying later their applicability in a concurrent process. Therefore, 17 racemic (hetero)aromatic sec-alcohols with different substitutions in the aromatic ring have been converted into enantioenriched amines with good to excellent selectivities (90–99% ee) and conversion values (67–99%). The scalability of the process was also demonstrated when two different amine donors were used in the transamination step, such as isopropylamine and cis-2-buten-1,4-diamine. Satisfyingly, both sacrificial amine donors can shift the equilibrium toward the amine formation, leading to the corresponding isolated enantioenriched amines with good to excellent results.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis


 Please wait while we load your content...
Please wait while we load your content...