Crown ether complexes of actinyls: a computational assessment of AnO2(15-crown-5)2+ (An = U, Np, Pu, Am, Cm)†
Abstract
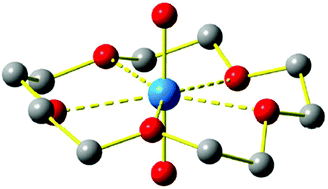
For further fundamental understanding of the nature and extent of covalency in actinyl–ligand bonding, and the benefits that this may have in the design of new ligands for nuclear waste separation, there is burgeoning interest in the nature of actinyl complexes with polydentate or multiple-point-donor ligands, such as crown ethers. There are few cases of structurally authenticated molecular actinyl–crown bonds under ambient conditions. We report here the computational characterization of AnO22+–(15-crown-5) complexes, where An = U, Np, Pu, Am, and Cm, and 15-crown-5 is the cyclic polyether ligand with five ether oxygen atoms. In the gas-phase complex, the actinyl group is located inside of the crown ether, tilted slightly out of the plane of the five equatorial oxygen atoms that coordinate the actinide metal center. The actinyl–cyclic ether complexes are found to exhibit a conventional conformation, with typical An–Oaxial and An–Oequatorial distances and angles. A striking result is the enhanced stability of the insertion complex for UO22+versus NpO22+, PuO22+, AmO22+ and CmO22+, which is evaluated in the context of An–O binding strengths (esp. bonding covalency), and may have ramifications for the utility of actinyl-crown complexes in separation applications.

 Please wait while we load your content...
Please wait while we load your content...