Open Access Article
Open Access ArticleFrom ethylzinc guanidinate to [Zn10O4]-supertetrahedron†
Michał K.
Leszczyński
a,
Iwona
Justyniak
a,
Karolina
Zelga
b and
Janusz
Lewiński
 *ab
*ab
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: lewin@ch.pw.edu.pl
bDepartment of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland
First published on 14th June 2017
Abstract
The controlled hydrolysis of an ethylzinc guanidinate complex affording an alkylzinc cluster containing a [Zn10O4]12+ supertetrahedron core stabilized by the guanidinate ligands is described. Accompanying investigations on the reactivity of this unprecedented cluster toward alcohols resulted in the formation of a mononuclear zinc alkoxide supported by the guanidinate ligands.
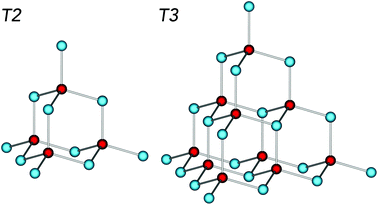
High nuclearity binary metal chalcogenide clusters are often regarded as boundary species between semiconductor nanomaterials and molecular compounds.1 These clusters incorporate ordered internal substructures of metal chalcogenides, and, unlike most nanocrystalline particles, can be prepared with atomic precision, which makes them excellent model compounds for the investigation of quantum confinement effects of semiconductors.2 One of the prevailing structural motifs of the metal chalcogenide clusters involves corner-sharing combination of tetrahedral M4X or MX4 (where most commonly M = Zn, Cd, In, Ga and X = S, Se, Te) units leading to the formation of supertetrahedral core structures (Fig. 1) stabilised by a variety of organic ligands.3 Moreover, the supertetrahedral clusters can be further assembled into extended periodic structures by the formation of direct covalent bonds4 (similarly to zeolites) or by the use of organic linkers5 (similarly to metal–organic frameworks) as well as by non-covalent interactions.6 The resulting superstructures involving clusters of uniform size can be applied as sensors and size- or shape-selective photocatalysts due to their well-defined crystalline porous structures.4,7 Similar cluster species incorporating μ4-O anions are often called polyoxometalates (POMs) and form a broad variety of structures (not only supertetrahedra) involving a range of metal centres.8
Despite the continual interest in ZnO-based nanomaterials, due to their intrinsic physicochemical properties which make them applicable to a broad range of top technologies, the chemistry of ZnO-based molecular clusters is still a relatively unexplored area.9,10 Since 1924, when the first tetrahedral [Zn4(μ4-O)] cluster coated by acetate ligands was synthesized,11 the structure of which was established in 1954,12 a large number of [Zn4O(L)6]-type molecular clusters stabilized by various organic ligands have been reported.9c,10,13 In turn, [Zn7(μ4-O)2]-type units involving two fused [Zn4(μ4-O)] tetrahedra or higher aggregates are much less common.9c,14 The formation of a [Zn10O4(OAc)12] cluster incorporating a supertetrahedral [Zn10O4]12+ core was also postulated15 but no example of the anticipated cluster has been isolated and structurally characterised until now.
As a part of our ongoing interest both in new molecular organozinc building blocks of inorganic–organic functional materials13g,h,16 and well-defined precursors of organic ligand-coated ZnO nanocrystals,17 herein we report on the controlled transformation of an ethylzinc guanidinate [EtZn(hpp)]3 (13) (where hpp = deprotonated 1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidine) leading to the isolation of a decanuclear organozinc aggregate [Et4Zn10O4(hpp)8] (2) which is the first well-defined cluster comprising the supertetrahedral [Zn10O4]12+ core stabilised by an organic ligand shell. We also endeavored to explore the reactivity of cluster 2 towards 1,1-diphenylethanol (Ph2CH3COH), which resulted in the formation of a new mononuclear zinc alkoxide [Zn(hppH)2(Ph2CH3CO)2] (3).
As we reported previously,18 the controlled aeration of 13 leads to the formation of the alkyl(oxo)zinc complex [EtZn(hpp)]4·ZnO (14·ZnO). This complex could also be obtained by the direct hydrolysis of 13 using 0.2 molar equivalents of H2O.18 In this vein, we wondered if the controlled hydrolysis of 13 using a higher molar ratio of water to the alkylzinc precursor could lead to the formation of higher oxo aggregates. Accordingly, the hydrolysis of 1 in THF solution using 0.4 molar equivalents of H2O was investigated, which reproducibly afforded a cluster [Et4Zn10O4(hpp)8] (2) (Scheme 1, path 1, for more details see the ESI, Fig. S1†). Compound 2 was characterised spectroscopically and its molecular structure was determined by the single crystal X-ray diffraction study (space group: P42/n). The molecular structure of 2 comprises a supertetrahedral [Zn10O4]12+ core stabilised by eight hpp guanidinate anions coordinated to Zn atoms in μ2- and μ3-bridging modes, forming a cluster of the S4 point group symmetry (Fig. 2 and Fig. S3†). The coordination sphere of the four apical zinc atoms in 2 is completed by the ethyl groups. Interestingly, the [Zn10O4]12+ core in 2 represents the ABC-type stacking of zinc atoms, which is typical of a metastable ZnO sphalerite phase. The Zn–O distances in the [Zn10O4]12+ core are within the range of 1.937(4) Å–1.983(4) Å, which is common for the [Zn4(μ4-O)]-type units,9a,b while Zn–O–Zn angles exhibit significant distortions from the ideal tetrahedral angle, which is probably related to the strain induced by the bridging guanidinate ligands (Table S1†).
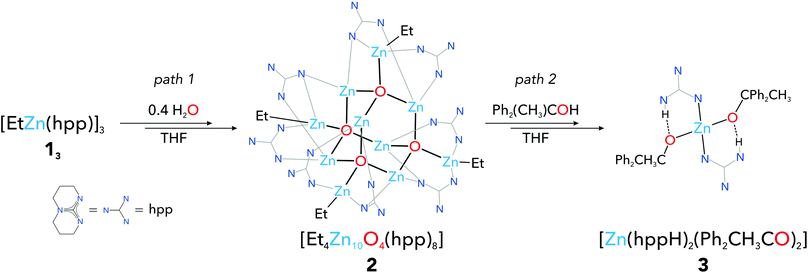 | ||
| Scheme 1 Synthesis of the [Zn10O4]-supertetrahedral cluster 2 from ethylzinc guanidinate (path 1), and its further reaction with an alcohol leading to 3 (path 2). | ||
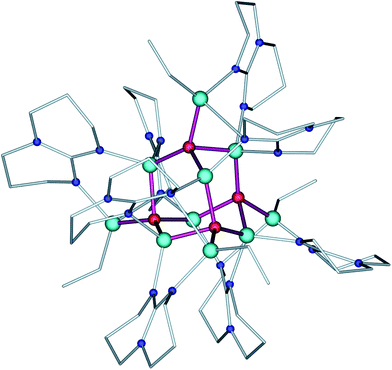 | ||
| Fig. 2 Molecular structure of 2. Zn = light blue, O = red, N = blue, C = grey, H atoms are omitted for clarity. Zn–O bonds forming the [Zn10O4]12+ core are depicted in purple. | ||
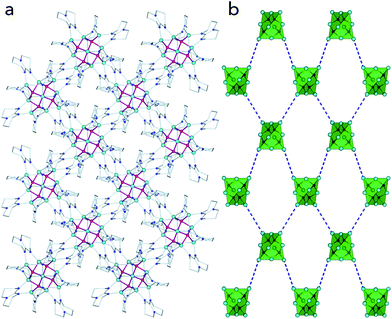
According to our recent reports, zinc clusters stabilised by bidentate monoanionic organic ligands (like carboxylates,13h quinolinates,16b or pyrrolylketiminates13e) often exhibit a unique propensity to the formation of microporous architectures mediated by non-covalent interaction-driven self-assembly processes. Indeed, the analysis of the crystal structure of 2 (Fig. 3a) revealed that the supertetrahedral clusters self-assemble into a diamondoid lattice with the apical Zn atoms facing each other for neighbouring clusters (Fig. 3b). However, the cavities observed within the crystal structure of 2 are too small for hosting any guest molecules.
The presence of ethylzinc groups in 2 potentially opens opportunities for post-synthetic modification of this cluster by the use of protic reagents. Inspired by the report of Wheatley demonstrating the modification of a methylzinc guanidinate cluster [Me2Zn3(hpp)4] mediated by Mes2BOH with the retention of the cluster core structure,19 we attempted transformations of 2 aiming at the preparation of new materials based on building blocks incorporating [Zn10O4]12+ cores. Therefore in the next step, we performed control reactions of 2 with dimesitylborinic acid (Mes2BOH) and 1,1-diphenylethanol (Ph2CH3COH) (for details, see the ESI†) While the reaction involving the borinic acid led to an intractable mixture of products, the use of the alcohol allowed for the isolation of a mononuclear complex [Zn(hppH)2(Ph2CH3CO)2] (3) (Scheme 1, path 2). Thus, in this case the transformation of cluster 2 to the mononuclear complex 3 upon reaction with Ph2CH3COH clearly indicated the decomposition of the supertetrahedral [Zn10O4]12+ core. Compound 3 was characterised spectroscopically and its molecular structure was determined by X-ray diffractometry (space group: P2/c) using single crystals grown from the concentrated THF solution. The molecular structure of 3 comprises a single zinc centre exhibiting a tetrahedral coordination sphere of two neutral hppH and two monoanionic Ph2CH3CO ligands (Fig. 4 and Fig. S4, Table S2†). In addition, the structure is stabilised by the intramolecular hydrogen bonds between the guanidine NH group and the alkoxide oxygen atoms. Similar structural features have previously been observed for zinc complexes with guanidines and O-donating ligands.19,20 Mononuclear zinc alkoxides similar to complex 3 are relatively rare due to their general tendency to aggregate into higher clusters.21 Surprisingly, despite several attempts we were not able to obtain complex 3 by the direct reaction of 1 with Ph2CH3COH, which yielded an amorphous solid (Fig. S2†) upon precipitation (see the ESI† for more details).
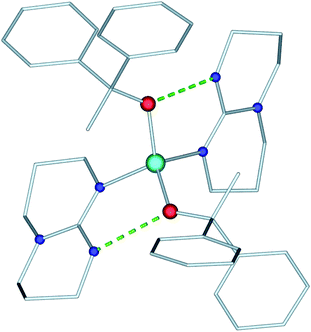 | ||
| Fig. 4 Molecular structure of 3. Zn = light blue, O = red, N = blue, C = grey, H atoms are omitted for clarity. NH–O hydrogen bonds have been depicted in green. | ||
In conclusion, we have developed the synthesis of unprecedented supertetrahedral [Zn10O4]12+ cluster stabilised by guanidinate ligands by the controlled hydrolysis of the [RZn(L)]-type precursor. Subsequent investigations exploring its reactivity resulted in the isolation and structure characterisation of a rare mononuclear form of a zinc alkoxide complex stabilized by guanidine ligands. Further studies on the preparation of organic ligand-coated supertetrahedral [Zn10O4]12+ clusters22 and their applications as molecular nano-ZnO models and predesigned synthons for MOF preparation using the mechanochemical approach10 are in progress.
The authors would like to acknowledge financial support from the Ministry of Science and Higher Education of Poland, under the project No. 0152/DIA/2012/41 within the Diamond Grant program as well as the National Science Centre – Grant No. 2014/13/B/ST5/04420.
Notes and references
- (a) J. F. Corrigan, O. Fuhr and D. Fenske, Adv. Mater., 2009, 21, 1867 CrossRef CAS; (b) B. M. Cossairt and J. S. Owen, Chem. Mater., 2011, 23, 3114 CrossRef CAS.
- (a) V. N. Soloviev, A. Eichhöfer, D. Fenske and U. Banin, J. Am. Chem. Soc., 2001, 123, 2354 CrossRef CAS PubMed; (b) H. Döllefeld, H. Weller and A. Eychmüller, J. Phys. Chem. B, 2002, 106, 5604 CrossRef; (c) B. M. Cossairt, P. Juhas, S. J. L. Billinge and J. S. Owen, J. Phys. Chem. Lett., 2011, 2, 3075 CrossRef CAS PubMed.
- (a) C.-C. Ou and C.-S. Yang, in Advanced Topics on Crystal Growth, InTech, 2013 Search PubMed; (b) G. Férey, Angew. Chem., Int. Ed., 2003, 42, 2576 CrossRef PubMed; (c) T. Wu, L. Wang, X. Bu, V. Chau and P. Feng, J. Am. Chem. Soc., 2010, 132, 10823 CrossRef CAS PubMed.
- (a) H. Li, A. Laine, M. O'Keeffe and O. M. Yaghi, Science, 1999, 283, 1145 CrossRef CAS PubMed; (b) N. Zheng, X. Bu, B. Wang and P. Feng, Science, 2002, 298, 2366 CrossRef CAS PubMed.
- (a) N. Zheng, X. Bu, H. Lu, L. Chen and P. Feng, J. Am. Chem. Soc., 2005, 127, 14990 CrossRef CAS PubMed; (b) N. Zheng, X. Bu, J. Lauda and P. Feng, Chem. Mater., 2006, 18, 4307 CrossRef CAS.
- (a) N. Zheng, X. Bu, H. Lu, Q. Zhang and P. Feng, J. Am. Chem. Soc., 2005, 127, 11963 CrossRef CAS PubMed; (b) N. Zheng, H. Lu, X. Bu and P. Feng, J. Am. Chem. Soc., 2006, 128, 4528 CrossRef CAS PubMed.
- N. Zheng, X. Bu and P. Feng, Nature, 2003, 426, 428 CrossRef CAS PubMed.
- (a) H. N. Miras, L. Vilà-Nadal and L. Cronin, Chem. Soc. Rev., 2014, 43, 5679 RSC; (b) H. N. Miras, J. Yan, D.-L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 RSC; (c) D.-L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS PubMed.
- (a) D. Prochowicz, K. Sokołowski and J. Lewiński, Coord. Chem. Rev., 2014, 270–271, 112 CrossRef CAS; (b) I. Haiduc, Coord. Chem. Rev., 2017, 338, 1 CrossRef CAS; (c) S. D. Pike, E. R. White, S. D. Pike, E. R. White, M. S. P. Shaffer and C. K. Williams, Nat. Commun., 2016, 7, 1 Search PubMed.
- For mechanochemical strategy of IRMOF assembly based on pre-designed [Zn4O(L)6]-type molecular clusters see: D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, Chem. Commun., 2015, 51, 4032 RSC.
- V. Auger and I. Robin, Compt. Rend., 1924, 178, 1546 CAS.
- H. Koyama and Y. Saito, Bull. Chem. Soc. Jpn., 1954, 27, 112 CrossRef CAS.
- For selected examples see: (a) A. Belforte, F. Calderazzo, U. Englert and J. Straehle, Inorg. Chem., 1991, 30, 3778 CrossRef CAS; (b) C. G. Lugmair, T. D. Tilley and A. L. Rheingold, Chem. Mater., 1997, 9, 339 CrossRef CAS; (c) J. Tao, M. L. Tong, J.-X. Shi, X.-M. Chen and S. W. Ng, Chem. Commun., 2000, 20, 2043 RSC; (d) J. Lewiński, W. Bury, M. Dutkiewicz, M. Maurin, I. Justyniak and J. Lipkowski, Angew. Chem., Int. Ed., 2008, 47, 573 CrossRef PubMed; (e) J. Lewiński, K. Suwała, T. Kaczorowski, M. Gałęzowski, D. T. Gryko, I. Justyniak and J. Lipkowski, Chem. Commun., 2009, 119, 215 RSC; (f) H. Khajavi, J. Gascon, J. M. Schins, L. D. A. Siebbeles and F. Kapteijn, J. Phys. Chem. C, 2011, 115, 12487 CrossRef CAS; (g) W. Bury, I. Justyniak, D. Prochowicz, Z. Wróbel and J. Lewiński, Chem. Commun., 2012, 48, 7362 RSC; (h) W. Bury, I. Justyniak, D. Prochowicz, A. Rola-Noworyta and J. Lewiński, Inorg. Chem., 2012, 51, 7410 CrossRef CAS PubMed.
- (a) R. Boomishankar, P. I. Richards and A. Steiner, Angew. Chem., Int. Ed., 2006, 45, 4632 CrossRef CAS PubMed; (b) P. I. Richards, R. Boomishankar and A. Steiner, J. Organomet. Chem., 2007, 692, 2773 CrossRef CAS; (c) A. F. Richards, A. Aprile and C. F. Hogan, Dalton Trans., 2012, 41, 8361 RSC.
- L. Spanhel, J. Sol-Gel Sci. Technol., 2006, 39, 7 CrossRef CAS.
- For selected examples, see: (a) J. Lewiński, M. Dranka, W. Bury, W. Śliwiński, I. Justyniak and J. Lipkowski, J. Am. Chem. Soc., 2007, 129, 3096 CrossRef PubMed; (b) K. Sokołowski, W. Bury, I. Justyniak, D. Fairén-Jiménez, K. Sołtys, D. Prochowicz, S. Yang, M. Schröder and J. Lewiński, Angew. Chem., Int. Ed., 2013, 52, 13414 CrossRef PubMed; (c) A. Grala, M. Wolska-Pietkiewicz, A. Wojewódzka, M. Dabergut, I. Justyniak and J. Lewiński, Organometallics, 2015, 34, 4959 CrossRef CAS; (d) K. Sokołowski, W. Bury, A. Tulewicz, A. M. Cieślak, I. Justyniak, D. Kubicki, E. Krajewska, A. Milet, R. Moszyński and J. Lewiński, Chem. – Eur. J., 2015, 21, 5496 CrossRef PubMed; (e) Z. Wróbel, I. Justyniak, I. Dranka and J. Lewiński, Dalton Trans., 2016, 45, 7240 RSC.
- (a) W. Bury, E. Krajewska, M. Dutkiewicz, K. Sokołowski, I. Justyniak, Z. Kaszkur, K. J. Kurzydłowski, T. Płociński and J. Lewiński, Chem. Commun., 2011, 47, 5467 RSC; (b) K. Sokołowski, I. Justyniak, W. Bury, J. Grzonka, Z. Kaszkur, Ł. Mąkolski, M. Dutkiewicz, A. Lewalska, D. Kubicki, K. Wójcik, K. Kurzydłowski and J. Lewiński, Chem. – Eur. J., 2015, 21, 5488 CrossRef PubMed; (c) P. Krupiński, A. Kornowicz, K. Sokołowski, A. M. Cieślak and J. Lewiński, Chem. – Eur. J., 2016, 22, 7817 CrossRef PubMed; (d) A. Grala, M. Wolska-Pietkiewicz, W. Danowski, Z. Wróbel, J. Grzonka and J. Lewiński, Chem. Commun., 2016, 52, 7340 RSC.
- K. Zelga, M. Leszczyński, I. Justyniak, A. Kornowicz, M. Cabaj, A. E. H. Wheatley and J. Lewiński, Dalton Trans., 2012, 41, 5934 RSC.
- S. J. Birch, S. R. Boss, S. C. Cole, M. P. Coles, R. Haigh, P. B. Hitchcock and A. E. H. Wheatley, Dalton Trans., 2004, 3568 RSC.
- M. P. Coles and P. B. Hitchcock, Eur. J. Inorg. Chem., 2004, 2662 CrossRef CAS.
- T. O. Petersen, S. Weigel, D. Kratzert, A. Fischer and I. Krossing, ChemistrySelect, 2017, 2, 265 CrossRef CAS.
- Remarkably, the [Zn10O4]-supertetrahedron core might be considered as a basic structural motif for a fractal-type arrangement, see: J. He, J.-X. Zhang, C.-K. Tsang, Z. Xu, Y.-G. Yin, D. Li and S.-W. Ng, Inorg. Chem., 2008, 47, 7948 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1545171 and 1545172. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7dt01619k |
| This journal is © The Royal Society of Chemistry 2017 |