Kinetic characterisation of a dye decolourising peroxidase from Streptomyces lividans: new insight into the mechanism of anthraquinone dye decolourisation
Abstract
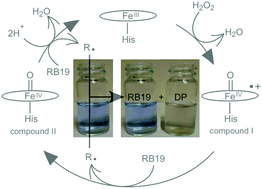
Dye decolourising peroxidases are the most recent family of haem peroxidases to be discovered. The oxidising potential of these enzymes is driven by the formation of ferryl intermediates that enables them to oxidise synthetic dye molecules that are widely used in the textile industry. We have investigated the catalytic cycle of a dye decolourising peroxidase (DtpA) from a biotechnologically important bacterium Streptomyces lividans. Using a combination of steady-state and stopped-flow kinetic investigations, we have determined the rate constants for all steps in the catalytic cycle with a range of substrate molecules. For most substrates, the value of kcat/Km measured by steady-state kinetics is equal to the slowest step in catalysis measured by stopped-flow spectroscopy, namely the decay of the ferryl FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species (compound II) to form the ferric species. With the anthraquinone-based dye, reactive blue 19 (RB19) unusual steady-state kinetic behaviour is observed, which we propose through kinetic modelling of the catalytic cycle is due to a disproportionation mechanism of the dye. At low RB19 concentrations, the rate of disproportionation is slower than that of the rate determining step in DtpA, whereas at higher concentrations of RB19 the rate of disproportionation is faster. This mechanism obviates the need to postulate secondary sites for substrate binding on the enzyme which has been previously proposed for other dye decolourising haem peroxidases.
O species (compound II) to form the ferric species. With the anthraquinone-based dye, reactive blue 19 (RB19) unusual steady-state kinetic behaviour is observed, which we propose through kinetic modelling of the catalytic cycle is due to a disproportionation mechanism of the dye. At low RB19 concentrations, the rate of disproportionation is slower than that of the rate determining step in DtpA, whereas at higher concentrations of RB19 the rate of disproportionation is faster. This mechanism obviates the need to postulate secondary sites for substrate binding on the enzyme which has been previously proposed for other dye decolourising haem peroxidases.


 Please wait while we load your content...
Please wait while we load your content...