Dicyanoaurate-based heterobimetallic uranyl coordination polymers†
Abstract
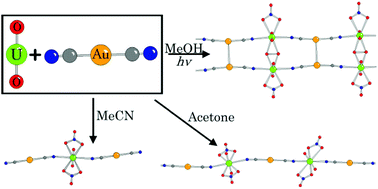
The first series of uranyl ([UO2]2+)-dicyanoaurate coordination polymers and molecular complexes has been synthesized. Reactions of [A][Au(CN)2] (A = [nBu4N]+ or [(Ph3P)2N]+ ([PPN])) and uranyl nitrate in alcoholic solvents in ambient light led to [A]2[(UO2)2(μ-η2:η2-O2)(NO3)2(μ-Au(CN)2)2], which incorporates peroxo ligands into a one-dimensional ladder topology with alternating aurophilic and peroxo rungs. Conducting the reaction with non-alcoholic solvents formed two polymorphs of a one-dimensional chain, [PPN][UO2(NO3)2Au(CN)2], from acetone, and a molecular analogue, [PPN]2[UO2(NO3)2(Au(CN)2)2], from acetonitrile, none of which exhibited aurophilic interactions. The addition of 2,2′-bipyridine to the initial reaction resulted in [UO2(bipy)(MeO)(MeOH)]2[(μ-Au(CN)2)(Au(CN)2)], a one-dimensional structure which propagates via a series of linear aurophilic bonds with pendant uranyl complexes; methanol and methoxy ligands provide additional connections through hydrogen bonding. The addition of 5,5′-dimethyl-2,2′-bipyridine using solvothermal conditions resulted in the one-dimensional ladder [UO2(Me2bipy)Au(CN)2]2[(μ-OH)2], generated through aurophilic bonds and hydroxide ligands. The incorporation of 2,2′:6′,2′′-terpyridine (terpy) using solvothermal conditions resulted in [[UO2(terpy)]2(μ-NO3)(μ-O)][Au(CN)2], a molecular salt with no aurophilic interactions. Emission spectra attributable to aurophilic interactions are observed in [nBu4N]2[(UO2)2(μ-η2:η2-O2)(NO3)2(μ-Au(CN)2)2], while all others only show emission typical of the uranyl cation.


 Please wait while we load your content...
Please wait while we load your content...