Kinetics and mechanism of oxidation of the anti-tubercular prodrug isoniazid and its analog by iridium(iv) as models for biological redox systems†
Abstract
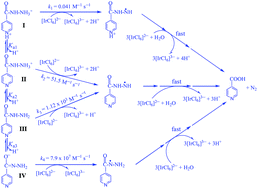
A complex reaction mechanism of oxidation of the anti-tubercular prodrug isoniazid (isonicotinic hydrazide, INH) by [IrCl6]2− as a model for redox processes of such drugs in biological systems has been studied in aqueous solution as a function of pH between 0 and 8.5. Similar experiments have been performed with its isomer nicotinic hydrazide (NH). All reactions are overall second-order, first-order in [IrCl6]2− and hydrazide, and the observed second-order rate constants k′ have been determined as a function of pH. Spectrophotometric titrations indicate a stoichiometry of [Ir(IV)] : [hydrazide] = 4 : 1. HPLC analysis shows that the oxidation product of INH is isonicotinic acid. The derived reaction mechanism, based on rate law, time-resolved spectra and stoichiometry, involves parallel attacks by [IrCl6]2− on all four protolytic species of INH and NH as rate-determining steps, depending on pH. These steps are proposed to generate two types of hydrazyl free radicals. These radicals react further in three rapid consecutive processes, leading to the final oxidation products. Rate constants for the rate-determining steps have been determined for all protolytic species I–IV of INH and NH. They are used to calculate reactivity–pH diagrams. These diagrams demonstrate that for both systems, species IV is ca. 105 times more reactive in the redox process than the predominant species III at the physiological pH of 7.4. Thus, species IV will be the main reactant, in spite of the fact that its concentration at this pH is extremely low, a fact that has not been considered in previous work. The results indicate that pH changes might be an important factor in the activation process of INH in biological systems also, and that in such systems this process most likely is more complicated than previously assumed.


 Please wait while we load your content...
Please wait while we load your content...