A theoretical study on oxidative cleavage of olefins to carbonyls catalysed by Fe(iii)-PyBisulidine†
Abstract
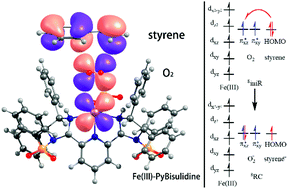
An environmentally friendly new protocol for the selective aerobic cleavage of styrene to carbonyl compounds using the Fe(III)-PyBisulidine catalyst has been reported recently. The catalyst features several unusual characteristics, such as its high efficiency lies on the ferric center instead of ferrous used by most iron-containing oxygenases and the catalyst specifically oxidizes phenyl-substituted olefins but exhibits no activity on nonconjugated olefins. Herein, we have investigated the mechanism of the oxidative cleavage reaction catalyzed by Fe(III)-PyBisulidine at the quantum chemistry level. Our computational study shows that the catalyst uses a dioxygen ligation mechanism to activate dioxygen to receive one electron from olefin, which triggers the oxidative cleavage reaction. Our study rationalizes that the Fe(II)-PyBisulidine catalyst is inactivated because ferrous is unable to raise the oxidizing ability of dioxygen. The exclusive oxidative cleavage of the phenyl-substituted olefin mainly results from the stability of the carbon cation, the orbital symmetry between the conjugated olefin and dioxygen, as well as a lower energy level of HOMO in conjugated olefin.


 Please wait while we load your content...
Please wait while we load your content...