Different catalytic behaviors of Pd and Pt metals in decalin dehydrogenation to naphthalene†
Abstract
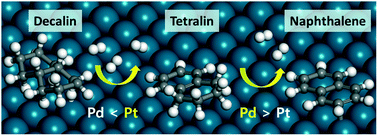
The catalytic dehydrogenation from decalin to tetralin to naphthalene is usually performed over supported Pd or Pt catalysts at a high temperature due to the endothermic nature of the reaction. However, the mechanistic studies of the catalytic activity and selectivity are not still sufficient to understand the dehydrogenation reaction on these metal surfaces. In this study, we mechanistically investigated the dehydrogenation reaction of decalin to tetralin to naphthalene on Pd and Pt catalysts using density functional theory (DFT) calculations combined with experimental validation. We firstly explored the relative energy profile of the entire elementary steps of the dehydrogenation reaction. Our theoretical results demonstrate that the conversion of decalin to tetralin on the Pt catalyst is energetically more preferred to that on Pd. On the other hand, Pd exhibits an energetically more favored reaction pathway in the conversion of tetralin to naphthalene than Pt. It is found that the difference in the catalytic activity and selectivity between Pd and Pt originates from the different structural and chemical characteristics of the metals. Our experimental results also support that decalin is more easily dehydrogenated over Pt/C while the dehydrogenation of tetralin is more facile over Pd/C.


 Please wait while we load your content...
Please wait while we load your content...