Structural motifs of water on metal oxide surfaces
Abstract
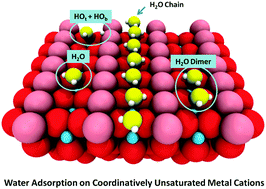
Understanding water/solid interactions is of great importance in a variety of fundamental and technological processes, such as photocatalytic water splitting, heterogeneous catalysis, electrochemistry, and corrosion. This review describes recent advancements in the molecular-level understanding of water adsorption, dissociation and clustering on model surfaces of metal oxides, achieved primarily by combining scanning probe microscopies with ensemble-averaged techniques and density functional theory calculations. Factors controlling how water binds and clusters on the coordinatively unsaturated metal cations of different oxide surfaces are discussed. We start by reviewing the fundamental differences in the relative stability of molecularly and dissociatively-bonded water monomers and clusters on isostructural rutile TiO2(110) and RuO2(110) surfaces and on different surfaces and polymorphs of TiO2. We further discuss how oxide interfaces (both exposed and buried) with metals affect water dissociation. Subsequently, we focus on high coverage water overlayers such as one-dimensional water chain structures that result from different substrate morphologies, and water monolayer structures. We conclude with novel studies of interfacial water in liquids.
- This article is part of the themed collection: Fundamental insights into interfacial catalysis


 Please wait while we load your content...
Please wait while we load your content...