The effect of cation mixing controlled by thermal treatment duration on the electrochemical stability of lithium transition-metal oxides†
Abstract
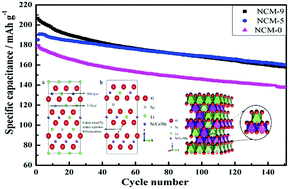
Lithium cathode materials have been considered as promising candidates for energy storage applications because of their high power/energy densities, low cost, and low toxicity. However, the Li/Ni cation mixing limits their application as practical electrode materials. The cation mixing of lithium transition-metal oxides, which was first considered only as the origin of performance degeneration, has recently been reconsidered as a way to stabilize the structure of active materials. Here we find that as the duration of the post-synthesis thermal treatment (at 500 °C) of LiNi1/3Co1/3Mn1/3O2 (NCM) was increased, the Li/Ni molar ratio in the final product was found to decrease, and this was attributed to the reduction in nickel occupying lithium sites; the cation mixing subtly changed; and those subtle variations remarkably influence their cycling performance. The cathode material with appropriate cation mixing exhibits a much slower voltage decay and capacity fade during long-term cycling. Combining X-ray diffraction, Rietveld analysis, the Fourier transform infrared technique, field-emission scanning electron microscopy, and electrochemical measurements, we demonstrate that an optimal degree of Ni2+ occupancy in the lithium layer enhances the electrochemical performance of layered NMC materials and that this occurs through a “pillaring” effect. The results provide new insights into “cation mixing” as a new concept for material design utilization of layered cathodes for lithium-ion batteries, thereby promoting their further application in lithium-ion batteries with new functions and properties.


 Please wait while we load your content...
Please wait while we load your content...