Revealing the distinct thermal transition behavior between PEGA-based linear polymers and their disulfide cross-linked nanogels†
Abstract
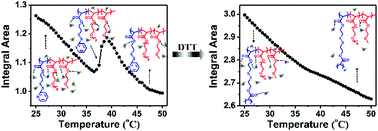
The distinct thermal transition behavior of thermoresponsive block polymers based on poly(ethylene glycol)methyl ether acrylate (PEGA) and their corresponding disulfide-cross-linked nanogels was studied by using FTIR measurements in combination with two-dimensional correlation spectroscopy (2Dcos). Spectral analysis clearly reveals that the sharp thermal transition of the precursor polymer is accompanied by a forced hydration process induced by the formation of hydrogen bonds between the entrapped water molecules and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, while the nanogel with a relatively continuous thermal transition is related to the existence of various dehydrating states of the C
O groups, while the nanogel with a relatively continuous thermal transition is related to the existence of various dehydrating states of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The C–H groups in the pyridyl disulfide (PDS) units exhibit a distinct change in the thermoresponsive profile of the precursor and the nanogel to show the effect of the polymer architecture on the thermal transition behavior. Additionally, a portion of the poly(N,N-dimethylacrylamide) (PDMA) segments is entrapped in the nanogel core, indicating that the thiol–disulfide exchange reaction occurs rapidly within the nanogels.
O groups. The C–H groups in the pyridyl disulfide (PDS) units exhibit a distinct change in the thermoresponsive profile of the precursor and the nanogel to show the effect of the polymer architecture on the thermal transition behavior. Additionally, a portion of the poly(N,N-dimethylacrylamide) (PDMA) segments is entrapped in the nanogel core, indicating that the thiol–disulfide exchange reaction occurs rapidly within the nanogels.


 Please wait while we load your content...
Please wait while we load your content...