Ionic effects on the proton transfer mechanism in aqueous solutions†
Abstract
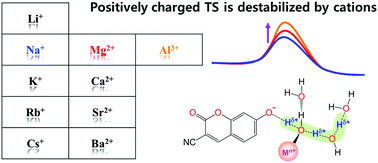
Proton dissociation (PD) reactions of weak acids and proton transfer (PT) processes in aqueous solutions are strongly influenced by ions. However, a detailed molecular picture that describes how ions affect the rates of PD and PT processes is still missing. Here, we utilize time-resolved fluorescence spectroscopy combined with quantum chemical calculations to investigate the excited-state proton transfer (ESPT) reaction of a photoacid in aqueous metal chloride solutions. The activation energy (Ea) for the ESPT of the photoacid increases with increasing charge density of cations (ρcat). The local hydrogen bond (H-bond) structure of the photoacid in the ionic hydration shell is strongly related to both the Ea and the ρcat. Most importantly, the proton's positive charge in the transition state, which is delocalized through the H-bonded water channel, is more destabilized with an increase in the ρcat, leading to a higher Ea. Our experimental and computational results allow us to elucidate the underlying mechanism for the ionic effect on PD and the subsequent PT process at the molecular level.


 Please wait while we load your content...
Please wait while we load your content...