Three coordination frameworks with copper formate based low dimensional motifs: synthesis, structure and magnetic properties†
Abstract
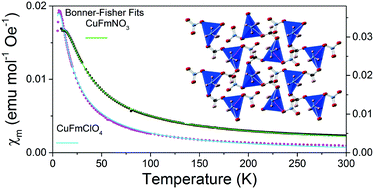
In this study we report the synthesis, crystal structures and magnetic properties of three frameworks wherein Cu cations are bridged by formate linkers into one-dimensional motifs. One of these compounds, Cu2(HCO2)3(C3N2H4)4(NO3), contains a ladder motif but remains paramagnetic to 2 K. This is likely because of the longer superexchange pathway along its chains due to the orientation of the Jahn–Teller axis of its Cu cations. In contrast Cu(HCO2)(NO3)(NH3)2 and Cu(HCO2)(ClO4)(NH3)2 feature Cu(HCO2) chains in which the Jahn–Teller axis is oriented perpendicular to the chain direction; these exhibit antiferromagnetic order below 12 and 7 K, respectively. Their magnetic susceptibilities are well fitted by a one-dimensional chain model but further examination of their magnetic properties reveals significant inter-chain magnetic coupling and a lack of spin dynamics. This suggests that these transitions correspond to the emergence of long-range magnetic order, highlighting the importance of detailed studies of frameworks containing low dimensional motifs to gain a deeper understanding of their magnetic behaviour.


 Please wait while we load your content...
Please wait while we load your content...