Catalytic transient leaving group for atom-economic synthesis of allenes from 2-alkynols†
Abstract
An atom economic approach from readily available propargylic alcohols to allenes, the first carboxylation of propargylic alcohols, has been established. Through the cooperative binary catalysis of Pd and a phosphoric acid, the reaction afforded multi-substituted allenoates with a broad scope tolerating useful functional groups. The synthetic potential of the obtained products has been demonstrated.
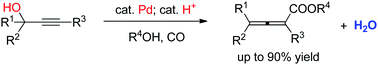


 Please wait while we load your content...
Please wait while we load your content...