Enantioselective gold(i)-catalyzed rearrangement of cyclopropyl-substituted 1,6-enynes into 2-oxocyclobutyl-cyclopentanes†
Abstract
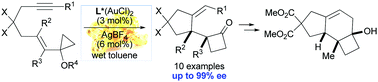
A gold(I)-catalyzed cycloisomerization/ring expansion sequence allows the highly enantioselective synthesis of 2-oxocyclobutylcyclopentane derivatives from cyclopropyl-substituted enynes. The bimetallic [(R)-MeO-DTBM-BIPHEP-(AuCl)2] complex was found to be the best precatalyst, affording the desired cyclobutanones in high yields and enantioselectivities (up to 99% ee). The usefulness of the method was further demonstrated by preparing the tricyclic core scaffold of russujaponol D.


 Please wait while we load your content...
Please wait while we load your content...