 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gender specific differences in the liver proteome of rats exposed to short term and low-concentration hexabromocyclododecane (HBCD)†
I.
Miller
a,
C.
Diepenbroek
b,
E.
Rijntjes
bc,
J.
Renaut
d,
K. J.
Teerds
b,
C.
Kwadijk
e,
S.
Cambier
d,
A. J.
Murk
f,
A. C.
Gutleb‡
d and
T.
Serchi‡
*d
aInstitute for Medical Biochemistry, Department for Biomedical Sciences, University of Veterinary Medicine Vienna, Veterinaerplatz 1, A-1210 Vienna, Austria. E-mail: ingrid.miller@vetmeduni.ac.at
bWageningen University, Human and Animal Physiology, P.O. Box 338, 6700 AH Wageningen, The Netherlands
cCharité-Universitätsmedizin Berlin, Institute of Experimental Endocrinology, Augustenburger Platz 1, 13353, Berlin, Germany
dEnvironmental Research and Innovation (ERIN) Department, Luxembourg Institute of Science and Technology (LIST), 5, avenue des Hauts-Fourneaux, L-4362 Esch-sur-Alzette, Grand-duchy of Luxembourg. E-mail: tommaso.serchi@list.lu; Tel: +352-470 261
eWageningen Institute for Marine Resources & Ecosystem Studies, IMARES, IJmuiden, The Netherlands
fWageningen University, Marine Animal Ecology Group, De Elst 1, 6708 WD Wageningen, The Netherlands
First published on 30th June 2016
Abstract
The influence of short term (7-day) exposure of male rats to the brominated flame retardant hexabromocyclododecane (HBCD) was studied by investigation of the liver proteome, both in euthyroid and hypothyroid rats and by comparing results with general data on animal physiology and thyroid hormone, leptin, insulin and gonadotropin concentrations determined in parallel. Proteome analysis of liver tissue by two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) revealed that only small protein pattern changes were induced by exposure in males, on just a few proteins with different functions and not involved in pathways in common. This is in contrast to previous findings in similarly exposed eu- and hypothyroid female rats, where general metabolic pathways had been shown to be affected. The largest gender-dependent effects concerned basal concentrations of liver proteins already in control and hypothyroid animals, involving mainly the pathways which were also differently affected by HBCD exposure. Among them were differences in lipid metabolism, which – upon exposure to HBCD – may also be the reason for the considerably higher ratio of γ-HBCD accumulated in white adipose tissue of exposed female rats compared to males. The results further elucidate the already suggested different sensitivity of genders towards HBCD exposure on the protein level, and confirm the need for undertaking toxicological animal experiments in both genders.
Introduction
Since 2013, the brominated flame retardant hexabromocyclododecane (HBCD) is banned in most European countries, but outside Europe still being produced and widely used (e.g. in the US). HBCD mainly serves as an additive in building industry (in polystyrene foams), but is also used for impregnation in equipment. Due to its persistent nature, HBCD is expected to remain present in food chains for several years to come. Like other substances of this chemical class, HBCD is lipophilic and accumulates in adipose tissue, not only in experimental animals but also in humans.1–5 While animal studies have shown low acute toxicity of HBCD, there are toxicological effects, especially in liver, on the immune system and on serum thyroid hormone (TH) dependent developmental processes as well as indications of endocrine disrupting properties.6–10 In cell culture, recently also an enhancing effect on cell migration and invasion has been described for this compound.11 Further elucidation of the underlying mechanisms of HBCD action would be needed for a better understanding of its adverse effects, and specific investigations applying modern analytical methods are likely to help in this task.Previous studies with 28d exposure of rats and variable doses of HBCD (between 0 and 200 mg per kg bw per day) revealed dose- and gender-dependent effects on body and organ weights as well as on TH levels, suggesting that female rats were more sensitive to exposure than male rats.8 In a similar experimental setup, transcriptomic data of rat liver and specific investigation of cytochrome P450 family members (CYPs) indicated gender-specific differences concerning affected genes/proteins related to metabolic pathways.6,7 Chronic exposure over longer intervals of time is likely to cause also secondary effects, which are likewise detected when screening for overall gene or protein level changes. Therefore, short term exposure may result in a different data set, better revealing primary effects of exposure to HBCD. Similarly, proteomic investigations are preferable to transcriptomic studies, as they show the actual protein concentrations in the organs under investigation.12,13
A recent HBCD short term (7 day) exposure study investigated the changes in the liver proteome of female rats and found a number of proteins differentially regulated in abundance, mainly related to general metabolic processes.10 In the present study, this investigation is extended to male rats and the experiment was performed under identical setup and conditions. This included euthyroid and hypothyroid animals as well as different HBCD exposure groups. Influences of dose, thyroid status and gender (including data from the previous experiment) on changes of the liver proteome are compared and further related to general animal data and hormone status determined in parallel. In the following, animal groups were named with an additional “m” and “f” depending on the gender (male, female) for easier comparison.
Animals, material and methods
Animals, treatment and experimental protocol
The animal experiment, incl. housing conditions and all experimental procedures, was approved under number 2006-051 by the Animal Welfare Committee of Wageningen University.The animal experiment was performed as the one described in detail,10 except that for the present investigation male euthyroid and hypothyroid rats were used instead of females. Hypothyroidism was induced during foetal life by feeding the dams an iodide-poor diet based on AIN 1993 requirements, supplemented with 0.75% sodium perchlorate to deplete endogenous iodide stores, as described in detail.14 After weaning the offspring continued on the same diet as the dams until the experimental intervention. In brief, 86-day-old male healthy euthyroid controls (mET) or hypothyroid (mHT) rats were fed 0, 3 or 30 mg of HBCD per kg body weight added to commercial custard pudding for 7 days. Group size was 6 animals (6 groups). At the termination of the experiment, blood was collected by heart puncture (5 IU heparin per ml blood), and animals were sacrificed by decapitation. Liver and white adipose tissues were collected, snap frozen and stored at −80 °C, plasma was prepared and stored at −20 °C.
Hormone measurements
Concentrations of total and free triiodothyronine (T3), corticosterone, leptin, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid stimulating hormone (TSH) were determined by radioimmunoassays (RIAs), as previously described.10,14 In addition, RIAs for total thyroxine (T4) (Diagnostic Systems Laboratories, Texas, USA) (DSL-3200), testosterone (DSL-4100), and insulin (Linco Research, St. Charles, USA) were performed according to the manufacturers’ protocols. The detection limits of these assays were: 5 ng ml−1 for total T4, 0.1 ng ml−1 for testosterone and 0.5 ng ml−1 for insulin. Intra- and interassay variations were determined using several pools of rat sera and were less than 10%.Determination of internal HBCD dose
Chemical analyses to determine the internal levels of HBCD were performed at IMARES, The Netherlands. In brief, adipose tissue samples spiked with 13C-labeled HBCD as internal standards. Samples were then extracted and α-, β- and γ-diastereomeres were determined by LC-ESI-MS as described.10Statistical analysis (for hormonal measurements)
Data is expressed as mean ± standard deviation (SD). Statistical analysis was carried out using SPSS 19.0 for Windows. Data was tested for normality using the Shapiro-Wilk test. If normality could not be assumed, data were LOG10 transformed. Group means were compared using a two-way Univariate Analysis of Variance (ANOVA). Correlation between internal γ-HBCD dose and other parameters was tested with the Spearman's rank correlation test. Values of P < 0.05 were considered to be significant.Proteomics
For the proteomic experiments, four liver samples per experimental group (mET and mHT; 0, 3 and 30 mg per kg per day HBCD, respectively) were randomly selected, subjected to two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) and further evaluated as previously described.10 In brief, rat livers were homogenized in lysis buffer (urea 7 M; thiourea 2 M; CHAPS 2% w/w; tris 30 mM, containing protease inhibitor) and proteins in the supernatant after centrifugation minimally labelled with CyDyes. Separation was performed on 24 cm IPGs of a non-linear 3–10 pH-range followed by SDS-PAGE in 12.5% gels, and images were captured on a Typhoon 9400 (DeCyder 7.0 software package, GE Healthcare, Diegem, Belgium). Gels were matched and subjected to univariate and multivariate analysis in order to determine differentially regulated spots with following criteria: fold change of at least 1.3 and P-value <0.05.10,15 These spots were automatically picked, tryptically digested and underwent MALDI-TOF/TOF analysis. Protein identification was performed by searching spectra against the SwissProt database with Rattus norvegicus as taxonomy, usually one PMF and up to 8 MS/MS spectra per spot. Parameters for the search were set as follow: up to two missed cleavages allowed, 100 ppm tolerance in PMF, 0.75 Da mass tolerance for precursor ion mass, carbamidomethyl cysteine as fixed modification, oxidation of methionine and oxidation of tryptophan (single oxidation, double oxidation and kynurenin) as variable modifications. Identifications were considered to be significant when the combined MOWSE score had P < 0.05. Criteria for positive MS identifications were in accordance with the Paris Report (http://www.mcponline.org/misc/ParisReport_Final.dtl).Statistics, including univariate analysis (ANOVA and t-test) and multivariate analysis (two-way ANOVA), was performed using the Extended Data Analysis (EDA) module, which is present inside the Decyder 7.0 software package.
Additional information on proteins (localization, function) and pathway analysis were retrieved from publicly available databases: UniProtKB database (http://www.uniprot.org/), STRING: functional protein association networks, v 9.1 (http://string-db.org/),16,17 for Rattus norvegicus proteins or genes, respectively, and for proteins with accession numbers from UniProt.
Results and discussion
Male rats
| HBCD-group | 0 | 3 | 30 | Thyroid effect (P-value) | HBCD effect (P-value) | Interaction (P-value) | Spearman's Rho | |
|---|---|---|---|---|---|---|---|---|
| <lod, below detection limit. n = 6 per group. Mean ± SD is given. lw, lipid weight. n.a., not analysed. ** indicates statistical significance. | ||||||||
| Body weight (g) | ET | 427 ± 36 | 428 ± 35 | 430 ± 27 | <0.001 | 0.828 | 0.736 | −0.045 |
| HT | 165 ± 22 | 153 ± 45 | 146 ± 32 | −0.272 | ||||
| Liver weight (g) | ET | 16.30 ± 1.12 | 16.14 ± 1.93 | 16.44 ± 1.68 | <0.001 | 0.867 | 0.766 | 0.058 |
| HT | 5.49 ± 0.80 | 5.12 ± 1.71 | 4.78 ± 1.11 | −0.295 | ||||
| TSH (ng ml−1) | ET | 0.38 ± 0.34 | 1.27 ± 0.92 | 0.49 ± 0.27 | <0.001 | 0.810 | 0.409 | 0.168 |
| HT | 17.01 ± 5.06 | 14.51 ± 4.31 | 16.76 ± 4.40 | −0.012 | ||||
| Total T4 (μg dl−1) | ET | 4.4 ± 0.3 | 3.9 ± 0.8 | 4.2 ± 0.5 | <0.001 | 0.189 | 0.229 | −0.306 |
| HT | <lod | <lod | <lod | 0.433 | ||||
| Total T3 (ng ml−1) | ET | 230 ± 48 | 234 ± 41 | 196 ± 9 | <0.001 | 0.189 | 0.587 | −0.35 |
| HT | 165 ± 12 | 177 ± 25 | 161 ± 47 | −0.148 | ||||
| Free T3 (pg ml−1) | ET | 5.95 ± 0.51 | 6.30 ± 0.61 | 5.27 ± 0.87 | <0.001 | 0.044 | 0.326 | −0.341 |
| HT | 1.56 ± 0.31 | 2.39 ± 0.78 | 1.80 ± 0.60 | 0.041 | ||||
| LH (ng ml−1) | ET | 0.61 ± 0.15 | 1.22 ± 0.51 | 0.88 ± 0.30 | 0.002 | 0.021 | 0.088 | 0.33 |
| HT | 0.54 ± 0.18 | 0.62 ± 0.28 | 0.55 ± 0.13 | 0.018 | ||||
| FSH (ng ml−1) | ET | 3.81 ± 0.43 | 5.04 ± 0.80 | 4.00 ± 0.83 | 0.065 | 0.039 | 0.491 | 0.161 |
| HT | 3.72 ± 1.15 | 4.16 ± 1.75 | 2.91 ± 0.83 | −0.359 | ||||
| Testosterone (ng ml−1) | ET | 2.23 ± 1.93 | 1.09 ± 0.36 | 1.24 ± 0.42 | 0.645 | 0.751 | 0.217 | −0.308 |
| HT | 0.98 ± 0.65 | 1.35 ± 1.45 | 1.64 ± 1.54 | 0.233 | ||||
| Leptin (ng ml−1) | ET | 8 ± 2 | 7 ± 2 | 8 ± 2 | 0.006 | 0.205 | 0.134 | 0.022 |
| HT | 13 ± 4 | 12 ± 4 | 8 ± 4 | −0.428 | ||||
| Insulin (ng ml−1) | ET | 6.02 ± 1.92 | 5.18 ± 2.69 | 8.81 ± 3.77 | <0.001 | 0.174 | 0.035 | 0.261 |
| HT | 4.38 ± 2.08 | 2.26 ± 1.48 | 2.09 ± 0.81 | −0.613** | ||||
| Corticosterone (ng ml−1) | ET | 149 ± 71 | 103 ± 62 | 222 ± 69 | 0.027 | 0.056 | 0.549 | 0.35 |
| HT | 183 ± 46 | 242 ± 113 | 353 ± 260 | 0.193 | ||||
| Internal γ-HBCD (mg per kg lw) | ET | <lod | 25.5 ± 5.2 | 81.0 ± 33.1 | 0.002 | <0.001 | 0.013 | |
| HT | <lod | 40.7 ± 8.6 | 130.0 ± 32.9 | |||||
Body weights and liver weights (given in Table 1) were both considerably smaller in mHT animals (similar to the literature report14), but did not differ between HBCD treated and non-treated groups of the same thyroid status.
Overall, the observed changes upon exposure to HBCD in mET animals were consistent with earlier reported HBCD effects on TH levels in the 28d study by van der Ven8 with ET rats. No studies have been reported with mHT rats.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22).
22).
 | ||
| Fig. 1 Two-dimensional gel image of a rat liver sample: 2D-DIGE separation of rat liver sample (master gel, grey level image). Marked spots show statistically significant changes in abundance upon HBCD exposure: 2 in mET animals (white squares), 6 in mHT rats (white triangles). In addition, 8 spots are of different abundance in ET and HT males (6 white diamonds + spots #1165, #1376 with other symbols). Detailed data on identifications is compiled in ESI Table 2.† | ||
| Significant changes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Effect of HBCD in ET-animals | |||||||||||
| Spot number | Protein name | UniProt ID | mET3/mET0 | mET30/mET0 | mET30/mET3 | Keywords/GO | Keywords/GO | Keywords/GO | |||
| Av. Ratio | T-test | Av. Ratio | T-test | Av. Ratio | T-test | Biological process | Cellular component | Molecular function | |||
| 1165 | rCG56002 | 0.877 | 0.548 | 1.310 | 0.135 | 1.490 | 0.0189 | ||||
| 1413 | Small glutamine-rich tetratricopeptide repeat-containing protein alpha | SGTA_RAT | 0.980 | 0.897 | 1.270 | 0.0527 | 1.300 | 0.00644 | Binds directly to HSC70 and HSP70 and | Cytoplasm | Chaperone |
| (b) Effect of HBCD in HT-animals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot number | Protein name | UniProt ID | mHT3/mHT0 | mHT30/mHT0 | mHT30/mHT3 | Keywords/GO | Keywords/GO | Keywords/GO | |||
| Av. Ratio | T-test | Av. Ratio | T-test | Av. Ratio | T-test | Biological process | Cellular component | Molecular function | |||
| 1226 | Formimidoyltransferase-cyclodeaminase | FTCD_RAT | 0.676 | 0.00852 | 0.800 | 0.0378 | 1.180 | 0.0753 | His metabolism | Cytoplasm, cytoskeleton, golgi | Folic acid binding, His catabolic process |
| 1357 | Serum paraoxonase/arylesterase 2 | PON2_RAT | 0.877 | 0.193 | 1.340 | 0.144 | 1.530 | 0.0436 | Aromatic compound catabolic process | Membrane | Arylesterase activity |
| 1376 | Serum paraoxonase/arylesterase 1 | PON1_RAT | 1.050 | 0.619 | 1.660 | 0.0501 | 1.580 | 0.0387 | Multiple regulation/response | Secreted, HDL | Hydrolase |
| 1502 | Guanidinoacetate N-methyltransferase | GAMT_RAT | 1.340 | 0.292 | 1.380 | 0.0477 | 1.030 | 0.708 | Biosynthetic/metabolic processes | Methyltransferase, transferase | |
| 1554 | Cytochrome b5 | CYB5_RAT | 0.658 | 0.0411 | 0.877 | 0.512 | 1.330 | 0.22 | Electron transport, transport | ER, membrane, microsome | Metal ion binding electron carrier activity |
| 1559 | Calmodulin | CALM_RAT | 1.560 | 0.041 | 1.390 | 0.341 | 0.885 | 0.588 | Multitude of regulations | Cytoplasm, cytoskeleton | Modulation by Ca++ |
| (c) Effect of TH status (same HBCD treatment) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spot number | Protein name | UniProt ID | mHT0/mET0 | mHT3/mET3 | mHT30/mET30 | Keywords/GO | Keywords/GO | Keywords/GO | |||
| Av. Ratio | T-test | Av. Ratio | T-test | Av. Ratio | T-test | Biological process | Cellular component | Molecular function | |||
| 1105 | Aldehyde dehydrogenase family 1 member L1 | AL1L1_RAT | 0.917 | 0.528 | 1.070 | 0.668 | 0.741 | 0.0407 | One-carbon metabolism | Cytoplasm | Oxidoreductase |
| 1107 | Aldehyde dehydrogenase family 1 member L1 | AL1L1_RAT | 0.877 | 0.625 | 1.080 | 0.729 | 0.595 | 0.0245 | One-carbon metabolism | Cytoplasm | Oxidoreductase |
| 1165 | rCG56002 | 0.847 | 0.46 | 1.350 | 0.0618 | 0.621 | 0.00384 | ||||
| 1271 | Succinate-semialdehyde dehydrogenase_mitochondrial | SSDH_RAT | 0.806 | 0.0326 | 0.763 | 0.0404 | 1.200 | 0.416 | Succinate metabolism, central nervous system | Mitochondrion | Oxidoreductase |
| 1289 | Keratin_type I cytoskeletal 18 | K1C18_RAT | 0.909 | 0.438 | 0.438 | 0.952 | 0.735 | 0.05 | Cytoplasm, intermediate filament, keratin nucleus | Structural molecule activity | |
| 1349 | Cystathionine gamma-lyase | CGL_RAT | 0.684 | 0.00202 | 1.070 | 0.82 | 0.820 | 0.655 | Amino acid biosynthesis, cys biosynthesis | Cytoplasm | Lyase |
| 1358 | Cystathionine gamma-lyase | CGL_RAT | 0.735 | 0.00429 | 1.000 | 0.989 | 0.820 | 0.545 | Amino acid biosynthesis, Cys biosynthesis | Cytoplasm | Lyase |
| 1395 | Serum paraoxonase/arylesterase 1 | PON1_RAT | 0.980 | 0.952 | 0.769 | 0.0394 | 1.130 | 0.305 | Multiple regulation/response | Secreted, HDL | Hydrolase |
In any of these group comparisons, pathway analyses failed to find connections between these few regulated proteins.
In summary, only very limited alterations of the liver proteome of male rats were observed between any of the exposed groups and thyroid status. Although a few significant changes of single proteins are noticed, they do not seem functionally connected, belonging to quite different pathways. The only protein family with more than one regulated member in these group comparisons are the PONs. One of their main functions is to metabolize toxic oxidized lipids in LDL (low-density lipoproteins) and HDL (high-density lipoproteins) particles, which may be created by environmental factors or drugs.24 The noticed small impacts on PON abundance in our experiments, in parallel to changes seen in leptin concentrations (Table 1), both dependent on HBCD exposure and/or thyroid status, may be interpreted as a small disturbance of lipid metabolism in the liver of male animals.
Comparison of changes in male and female animals
A previous study from our groups has reported proteomic data as well as hormonal and general animal data in an identical experimental setup with female rats10 and performed in parallel. This allows the comparison with the here presented data of males in order to filter for gender specific effects.General animal and hormone data of animals of both genders are summarized in ESI Table 1† and show the expected differences in body weight, liver weight and gonadotropins between males and females. Especially in ET status, male rats accumulate much less HBCD in adipose tissue compared to female animals (gender effect P = 0.001). Van der Ven et al.8 also reported a lower accumulation of γ-HBCD in liver lipid for mET than for fET rat. However, a major difference between the present study and the investigation by van der Ven and colleagues is that most of their animals received much higher doses of HBCD (up to 200 mg per kg bw per day), and the overall exposure time was 4 times longer compared to the present study. This resulted in changes in liver weights of females, an observation that was not confirmed in our studies, neither for ET nor for HT animals.
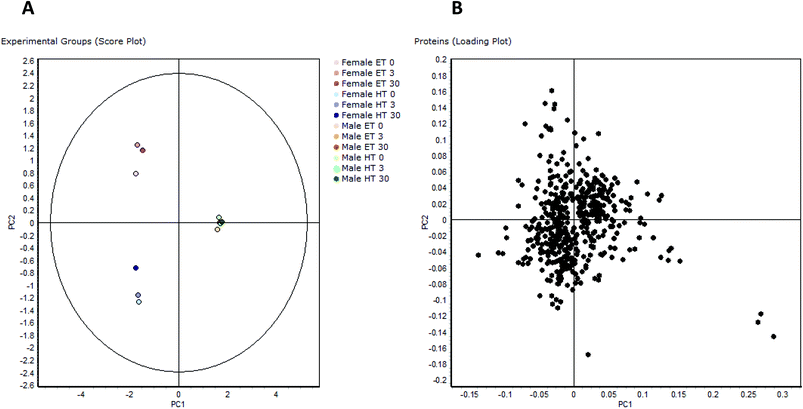 | ||
| Fig. 2 PCA of proteomic data: PCA of the 496 differentially regulated protein spots: (A) comparison of experimental groups (score plot), (B) comparison of all proteins (loading plot). | ||
The loading plot of the 496 regulated spots visualizes similarity of single spot abundance between PC1 and PC2, and most spots group in a compact cluster (Fig. 2b). The distance of the spots from the centre of the cluster reflects their similarity in regulation behaviour. Some spots/proteins at the border area of the cluster are the ones that had already been found regulated in female rats depending on thyroid status (e.g. glutathione-S-transferases10), but also some typical serum proteins with known functions as protease inhibitors or reactants in inflammation, being known for their concentration differences between genders.25 The spots farthest from the centre are those with most different behaviour between PC1 and PC2, i.e. three spots in the lower right, belonging to the protein carbonic anhydrase 3. Carbonic anhydrase 3 is known as a growth hormone-dependent liver enzyme, its concentration is gender-dependent, and changed in diabetes/obesity.26–29 It is one of the proteins already noticed in a previous paper as consistently increased in fHT liver.10 Frémont et al.29 have reported previously the presence of a connection between thyroid status and carbonic anhydrase 3 concentration in skeletal muscle. Gender-dependent abundance differences noticed in this study are even higher, more than 10-fold in mET and more than 5-fold in mHT rats with comparable HBCD exposure, and all highly significant.
Gender-influence in response towards HBCD exposure has been shown on the transcriptomic level, in a study with a much longer exposure time and different doses (most of them much higher than ours), in ET rats.6 In the study by Cantón6 in females genes related to lipid metabolism, triacylglycerol and cholesterol metabolism were markedly downregulated. In contrast, genes involved in phase I and II metabolism were found upregulated, especially in males, which, for the authors, represented the reason of the lower susceptibility to HBCD of males as compared to females. The described changes were more obvious at higher HBCD doses, and at doses similar to our study, the overall differences between genders and between exposed and unexposed were rather limited. In our study, investigating the proteome, and after the one week exposure, we detected only minor changes in male rats, limited to very few singular proteins. Compared to previous reports, this is a much shorter exposure time, and a time delay between gene regulation and protein synthesis has to be expected. Still, in an identical experiment with females rats10 changes in lipid and carbohydrate metabolism were clearly visible, which is in accordance with the transcriptomics results reported by Cantón et al.6
The only other proteomic study that involved HBCD (administered in a mixture with other flame retardants) and determined its impact on the liver proteome was performed in zebrafish.30 Though different to the present study in many main parameters (species, mixed compound, exposure time), it also reported gender-specific effects.
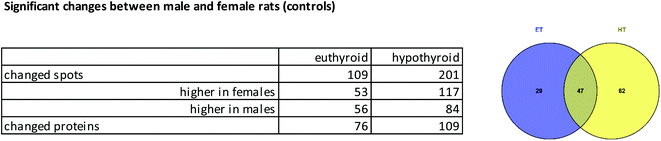 | ||
| Fig. 3 Spot differences between genders: numbers of spots or proteins with altered abundance between male and female controls, as a table and Venn diagram (for proteins). | ||
Grouping of these spots and network analysis put the vast majority of these proteins in connection with metabolic processes (ESI Table 3†). For ET animals, there were strong clusters of differentially regulated proteins between genders related to cytochrome P450 (drug metabolism, metabolism of xenobiotics) and glutathione metabolism. Many more members of these protein families were represented in ET animals than in HT animals. In HT animals quite a number of proteins from the fatty acid metabolism were differentially regulated between male and female rats (ESI Table 3,†Fig. 4). Comparison of Fig. 4a and b shows gender differences in the number of interacting proteins, influenced by thyroid status.
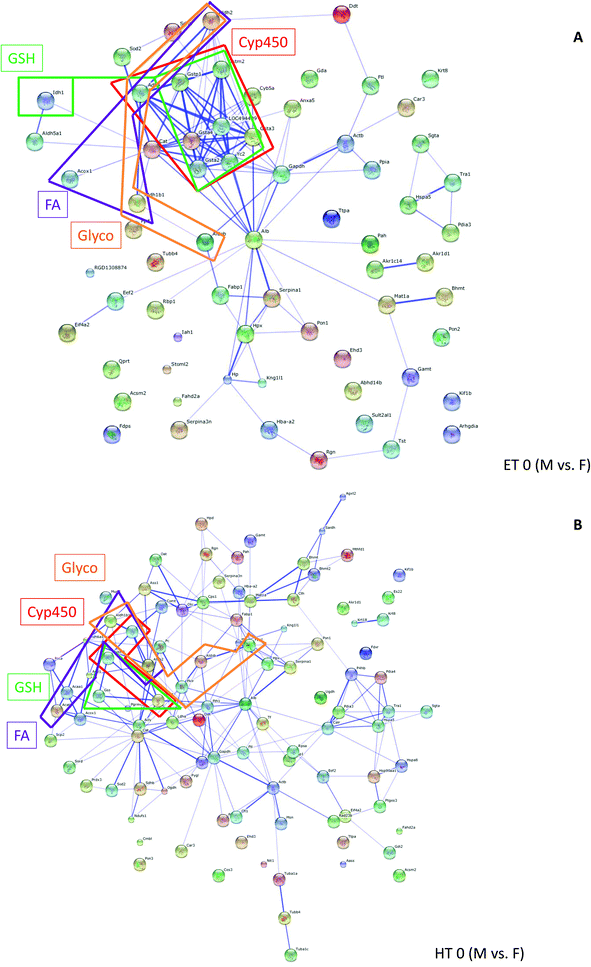 | ||
| Fig. 4 Pathway analysis (STRING): networks created from proteins differentially regulated in male and female rats (controls) in ET animals (A) or HT rats (B). Members from pathways related to Glycolysis/Gluconeogenesis (Glyco), Fatty acid metabolism (FA), Drug metabolism or Metabolism of xenobiotics by cytochrome P450 (CYP), Glutathione metabolism (GSH) are boxed. Boxes are displayed in colour. Further details on pathways and gene/protein names are compiled in ESI Table 3.† | ||
Several gender-specific differences in lipid metabolism have been reported earlier, e.g. in rats fed a high fat diet different strategies to maintain energetic and metabolic homeostasis in response to feeding have been elicited,31,32 in the liver proteome,33 as well as in plasma proteins34 and in a rat model of alcoholic steatohepatitis.35 These reports on gender-dependency in lipid metabolism confirm our proteomic results. We noticed small changes in the leptin levels, which were significant in gender*thyroid status interaction, but not in gender effect alone (MANOVA 0.167). Still, gender-related differences in lipid metabolism may also be the reason for the gender differences in HBCD accumulation in adipose tissue in our rats (ESI Table 1†). Studies on liver mitochondrial oxidative metabolism showed gender dimorphism as well,36–38 namely a different profile of CYP isoforms in microsomes.7,39,40 In our study we could not detect any differentially regulated CYP proteins, probably due to the choice of analysing complete, unfractionated liver lysates, however pathway analysis (Fig. 4, ESI Table 3†) clearly filtered out gender-specific differences in proteins connected to metabolism of xenobiotics/drug metabolism, specifically members of the GST family.
Interestingly, the observed gender-specifically regulated pathways are also the ones affected by HBCD exposure in a gender specific way in the present (and previous) study. Also other reports have shown differences in gender-susceptibility (in rat serum proteins in health and inflammation,25,41 in mouse proteins in muscle42 or in the aging kidney43). Our results are thus in line with a recent NIH directive/recommendation for gender-balance in further biomedical research studies.44,45
Conclusions
In male rats exposed to levels up to 30 mg per kg HBCD during 7 days, only limited changes in liver protein content and plasma hormone concentrations could be observed. This is in clear contrast to previous findings in female rats10 using the same model. Based on pathway analysis involving the whole set of proteins separated and identified by this proteomic study, we could attribute these differences to alterations in metabolism in male and female rats, both under ET and HT conditions, mainly in lipid metabolism and glycolysis/gluconeogenesis, but also in redox and CYP protein related responses. This supports the need to include both genders in animal experiments.Proteomics proved its usefulness in toxicological investigations, detecting (i) first signs of dysregulation on the protein level after only one week of HBCD exposure, (ii) protein pattern differences in female rats based on thyroid status and – as a main influence – (iii) gender-dependent protein regulation of metabolic pathways, which have a major impact on the body's reactivity towards noxious substances.
Funding information
This work was supported by contributions from the participating institutions.Conflict of interest statement
The authors do not declare any conflict of interest.Abbreviations
| 2D-DIGE | Two-dimensional fluorescence difference gel electrophoresis |
| CYP | Cytochrome P450 |
| ET | Euthyroid |
| f | Female |
| FSH | Follicle-stimulating hormone |
| GO | Gene ontology |
| HBCD | Hexabromocyclododecane |
| HT | Hypothyroid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC | Liquid chromatography |
| m | Male |
| MS | Mass spectrometry |
| PMF | Peptide mass fingerprint |
| PON | Paraoxonase/arylesterase |
| RIA | Radioimmunoassay |
| STRING | Functional protein association networks |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TH | Thyroid hormone |
| TSH | Thyroid stimulating hormone |
Acknowledgements
We would like to thank Hans van den Berg and Hans Swarts for their help with the animal experiments.References
- A. Covaci, A. C. Gerecke, R. J. Law, S. Voorspoels, M. Kohler, N. V. Heeb, H. Leslie, C. R. Allchin and J. De Boer, Hexabromocyclododecanes (HBCDs) in the environment and humans: A review, Environ. Sci. Technol., 2006, 40, 3679–3688 CrossRef CAS PubMed.
- P. O. Darnerud, Toxic effects of brominated flame retardants in man and in wildlife, Environ. Int., 2003, 29, 841–853 CrossRef CAS PubMed.
- C. A. De Wit, An overview of brominated flame retardants in the environment, Chemosphere, 2002, 46, 583–624 CrossRef CAS PubMed.
- K. Kalachova, P. Hradkova, D. Lankova, J. Hajslova and J. Pulkrabova, Occurrence of brominated flame retardants in household and car dust from the Czech Republic, Sci. Total Environ., 2012, 441, 182–193 CrossRef CAS PubMed.
- G. Tomko and K. M. McDonald, Environmental fate of hexabromocyclododecane from a new Canadian electronic recycling facility, J. Environ. Manage., 2013, 114, 324–327 CrossRef CAS PubMed.
- R. F. Cantón, A. A. Peijnenburg, R. L. Hoogenboom, A. H. Piersma, L. T. Van der Ven, M. Van den Berg and M. Heneweer, Subacute effects of hexabromocyclododecane (HBCD) on hepatic gene expression profiles in rats, Toxicol. Appl. Pharmacol., 2008, 231, 267–272 CrossRef PubMed.
- S. Germer, A. H. Piersma, L. van der Ven, A. Kamyschnikow, Y. Fery, H.-J. Schmitz and D. Schrenk, Subacute effects of the brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on hepatic cytochrome P450 levels in rats, Toxicology, 2006, 218, 229–236 CrossRef CAS PubMed.
- L. T. M. Van der Ven, P. E. G. Leonards, W. Slob, H. Lilienthal, S. Litens, M. Herlin, H. Håkansson, R. F. Cantón, M. Van den Berg, T. J. Visser, H. van Loveren, J. G. Vos and A. H. Piersma, A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats, Toxicol. Sci., 2006, 94, 281–292 CrossRef CAS PubMed.
- L. T. M. Van der Ven, T. van de Kuil, P. E. G. Leonards, W. Slob, H. Lilienthal, S. Litens, M. Herlin, H. Håkansson, R. F. Cantón, M. Van den Berg, T. J. Visser, H. van Loveren, J. G. Vos and A. H. Piersma, Endocrine effects of of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats, Toxicol. Lett., 2009, 185, 51–62 CrossRef CAS PubMed.
- I. Miller, T. Serchi, S. Cambier, C. Diepenbroek, J. Renaut, J. H. J. Van den Berg, C. Kwadijk, A. C. Gutleb, E. Rijntjes and A. J. Murk, Hexabromocyclododecane (HBCD) induced changes in the liver proteome of eu- and hypothyroid female rats, Toxicol. Lett., 2016, 245, 40–51 CrossRef CAS PubMed.
- Y. Zhong, C. Chen, X. Wang, P. Guo, X. Zhang, Z. Yu and J. An, HBCD and PCBs enhance the cell migration and invasion of HepG2 via the PI3 K/Akt pathway, Toxicol. Res., 2015, 4, 677–685 RSC.
- D. Greenbaum, C. Colangelo, K. Williams and M. Gerstein, Comparing protein abundance and mRNA expression levels on a genomic scale, BMC Genome Biol., 2003, 4, 117, DOI:10.1186/gb-2003-4-9-117.
- Q. Tian, S. B. Stepaniants, M. Mao, L. Weng, M. C. Feetham, M. J. Doyle, E. C. Yi, H. Dai, V. Thorsson, J. Eng, D. Goodlett, J. P. Berger, B. Gunter, P. S. Linseley, R. B. Stoughton, R. Aebersold, S. J. Collins, W. A. Hanlon and L. E. Hood, Integrated Genomic and Proteomic Analyses of Gene Expression in Mammalian Cells, Mol. Cell. Proteomics, 2004, 3, 960–969 CAS.
- E. Rijntjes, H. J. M. Swarts, E. Anand-Ivell and K. J. Teerds, Prenatal induced chronic dietary hypothyroidism delays but does not block adult-type Leydig cell development, Am. J. Physiol.: Endocrinol. Metab., 2009, 296, E305–E314 CrossRef CAS PubMed.
- A. Alban, S. O. David, L. Bjoreksten, C. Andersson, E. Sloge, S. Lewis and I. Currie, A novel experimental design for comparative two-dimensional analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard, Proteomics, 2003, 3, 36–41 CrossRef CAS PubMed.
- B. Snel, G. Lehmann, P. Bork and M. A. Huynen, STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene, Nucleic Acids Res., 2000, 28, 3442–3444 CrossRef CAS PubMed.
- A. Franceschini, D. Szklarczyk, S. Frankild, M. Kuhn, M. Simonovic, A. Roth, J. Lin, P. Minguez, P. Bork, C. von Mering and L. J. Jensen, STRING v9.1: protein–protein interaction networks, with increased coverage and integration, Nucleic Acids Res., 2013, 41, D808–D815 CrossRef CAS PubMed.
- H. F. Escobar-Morreale, F. Escobar del Rey and G. Morreale de Escobar, Thyroid hormones influence serum leptin concentrations in the rat, Endocrinology, 1997, 138, 4485–4488 CrossRef CAS PubMed.
- G. R. Hynes and P. J. Jones, Leptin and its role in lipid metabolism, Curr. Opin. Lipidol., 2001, 12, 321–327 CrossRef CAS PubMed.
- M. Schriks, C. M. Vrabie, A. C. Gutleb, E. Faassen, I. M. C. M. Rietjens and A. J. Murk, T-screen to quantify potentiating, antagonistic and thyroid hormone-like activities of polyhalogenated aromatic hydrocarbons (PHAHs), Toxicol. In Vitro, 2006, 20, 490–498 CrossRef CAS PubMed.
- M. Schriks, E. Zvinavashe, J. D. Furlow and A. J. Murk, Disruption of thyroid hormone-mediated Xenopus laevis tadpole tail tip regression by hexabromocyclododecane (HBCD) and 2,2′,3,3′,4,4′,5,5′,6-nona brominated diphenyl ether (BDE206), Chemosphere, 2006, 65, 1904–1908 CrossRef CAS PubMed.
- I. Miller, J. Renaut, S. Cambier, A. J. Murk, A. C. Gutleb and T. Serchi, Dataset of liver proteins of eu- and hypothyroid rats affected in abundance by any of three factors: in vivo exposure to hexabromocyclododecane (HBCD), thyroid status, gender differences, Data-in-brief Search PubMed , submitted in parallel.
- H. L. Li, D. P. Liu and C. C. Liang, Paraoxonase gene polymorphisms, oxidative stress, and diseases, J. Mol. Med., 2003, 81, 766–779 CrossRef CAS PubMed.
- L. G. Costa, A. Vitalone, T. B. Cole and C. E. Furlong, Modulation of paraoxonase (PON1) activity, Biochem. Pharmacol., 2005, 69, 541–550 CrossRef CAS PubMed.
- I. Miller, P. Haynes, I. Eberini, M. Gemeiner, R. Aebersold and E. Gianazza, Proteins of Rat Serum: III. Gender-related differences in protein concentration under baseline conditions and upon experimental inflammation as evaluated by two-dimensional electrophoresis, Electrophoresis, 1999, 20, 836–845 CrossRef CAS PubMed.
- A. Shiels, S. Jeffery, I. R. Phillips, E. A. Shephard, C. A. Wilson and N. D. Carter, Sexual differentiation of rat liver carbonic anhydrase III, Biochim. Biophys. Acta, 1983, 760, 335–342 CrossRef CAS.
- C. J. Lynch, W. A. Brennan Jr., T. C. Vary, N. Carter and S. J. Dodgson, Carbonic anhydrase III in obese Zucker rats, Am. J. Physiol., 1993, 264, E621–E630 CAS.
- T. Nishita, S. Igarashi and M. Asari, Determination of carbonic anhydrase-III by enzyme-immunoassay in liver, muscle and serum of male rats with streptozotocin-induced diabetes mellitus, Int. J. Biochem. Cell Biol., 1995, 27, 359–364 CrossRef CAS PubMed.
- P. Frémont, C. Lazure, R. R. Tremblay, M. Chrétien and P. A. Rogers, Regulation of carbonic anhydrase III by thyroid hormone: opposite modulation in slow- and fast-twitch skeletal muscle, Biochem. Cell. Biol., 1987, 65, 790–797 CrossRef.
- P. Kling, A. Norman, P. L. Andersson, L. Norrgren and L. Förlin, Gender-specific proteomic responses in zebrafish liver following exposure to a selected mixture of brominated flame retardants, Ecotoxicol. Environ. Saf., 2008, 71, 319–327 CrossRef CAS PubMed.
- E. Amengual-Cladera, I. Lladó, M. Gianotti and A. M. Proenza, Sex differences in the effect of high-fat diet feeding on rat white adipose tissue mitochondrial function and insulin sensitivity, Metab., Clin. Exp., 2012, 61, 1108–1117 CrossRef CAS PubMed.
- A. Nadal-Casellas, A. Amengual-Cladera, A. M. Proenza, I. Lladó and M. Gianotti, Long-term High-fat-diet Feeding Impairs Mitochondrial Biogenesis in Liver of Male and Female Rats, Cell. Physiol. Biochem., 2010, 26, 291–302 CrossRef CAS PubMed.
- X. Wang, J. W. Choi, T. S. Oh, D. K. Choi, R. Mukherjee, H. Liu and J. W. Yun, Comparative hepatic proteome analysis between lean and obese rats fed a high-fat diet reveals the existence of gender differences, Proteomics, 2012, 12, 284–299 CrossRef CAS PubMed.
- H. Liu, J.-W. Choi and J. W. Yun, Gender differences in rat plasma proteome in response to high-fat diet, Proteomics, 2012, 12, 269–283 CrossRef CAS PubMed.
- A. Banerjee, W. K. Russell, A. Jayaraman and S. K. Ramaiah, Identification of proteins to predict the molecular basis for the observed gender susceptibility in a rat model of alcoholic steatohepatitis by 2-D gel proteomics, Proteomics, 2008, 8, 4327–4337 CrossRef CAS PubMed.
- C. Borras, J. Sastre, D. Garcia-Sala, A. Lloret, F. V. Pallardo and J. Vina, Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males, Free Radical Biol. Med., 2003, 34, 546–552 CrossRef CAS PubMed.
- N. Ståhlberg, E. Rico-Bautista, R. M. Fisher, X. Wu, L. Cheung, A. Flores-Morales, G. Tybring, G. Norstedt and P. Tollet-Egnell, Female-Predominant Expression of Fatty Acid Translocase/CD36 in Rat and Human Liver, Endocrinology, 2004, 145, 1972–1979 CrossRef PubMed.
- R. Justo, J. Boada, M. Frontera, J. Oliver, J. Bermudez and M. Gianotti, Gender dimorphism in rat liver mitochondrial oxidative metabolism and biogenesis, Am. J. Physiol.: Cell Physiol., 2005, 289, C372–C378 CrossRef CAS PubMed.
- S. Nisar, C. S. Lane, A. F. Wilderspin, K. J. Welham, W. J. Griffiths and L. H. Patterson, A proteomic approach to the identification of cytochrome P450 isoforms in male and female rat liver by nanoscale liquid chromatography-electrospray ionization-tandem mass spectrometry, Drug Metab. Dispos., 2004, 32, 382–386 CrossRef CAS PubMed.
- H. J. Huang, M. L. Tsai, Y. W. Chen and S. H. Chen, Quantitative shot-gun proteomics and MS-based activity assay for revealing gender differences in enzyme contents for rat liver microsome, J. Proteomics, 2011, 74, 2734–2744 CrossRef CAS PubMed.
- R. Ballerio, E. Gianazza, L. Mussoni, I. Miller, P. Gelosa, U. Guerrini, I. Eberini, M. Gemeiner, S. Belcredito, E. Tremoli and L. Sironi, Gender differences in endothelial function and inflammatory markers along the occurrence of pathological events in stroke-prone rats, Exp. Mol. Pathol., 2007, 82, 33–41 CrossRef CAS PubMed.
- K. Dimova, L. A. Metskas, M. Kulp and S. P. Scordilis, Skeletal muscle gender dimorphism from proteomics, J. Visualized Exp., 2011, 58, e3536, DOI:10.3791/3536.
- H. Amelina and S. Cristobal, Proteomic study on gender differences in aging kidney of mice, Proteome Sci., 2009, 7, 16, DOI:10.1186/1477-5956-7-16.
- J. A. Clayton and F. S. Collins, NIH to balance sex in cell and animal studies, Nature, 2014, 509, 282–283 CrossRef PubMed.
- J. A. Clayton, Studying both sexes: a guiding principle for biomedicine, FASEB J., 2016, 30, 519–524 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tx00166a |
| ‡ Shared Senior Authorship. |
| This journal is © The Royal Society of Chemistry 2016 |
