Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Developing Mn-doped lead sulfide quantum dots for MRI labels†
Lyudmila
Turyanska
*ab,
Fabrizio
Moro
a,
Amalia
Patanè
a,
James
Barr
a,
Walter
Köckenberger
a,
Alexander
Taylor
c,
Henryk M.
Faas
c,
Maxine
Fowler
c,
Peter
Wigmore
c,
Rebecca C.
Trueman
c,
Huw E. L.
Williams
d and
Neil R.
Thomas
d
aSchool of Physics and Astronomy, The University of Nottingham, NG72RD, UK. E-mail: Lyudmila.Turyanska@nottingham.ac.uk
bSchool of Chemistry, University of Lincoln, LN6 7TS, UK
cFaculty of Medicine and Health Sciences, The University of Nottingham, NG72RD, UK
dCentre for Biomolecular Sciences, School of Chemistry, The University of Nottingham, NG72RD, UK
First published on 10th October 2016
Abstract
Magnetic interactions of Mn2+ ions in lead sulfide (PbS) nanocrystals with protons in water are probed by NMR and MRI. A thin layer of capping molecules enables free solvent diffusion to the nanocrystal surface resulting in a decrease of proton relaxation times. Magnetic resonance imaging of neuronal cell pellets exposed to (PbMn)S at non-toxic concentrations demonstrates their prospects as MRI-labels.
Doping of colloidal semiconductor nanocrystals (Quantum Dots, QDs) with impurities has the potential to advance the use of QDs in a range of applications from optoelectronic devices to biomedical imaging.1,2 The optical stability and tuneability of the QD emission offer significant advantages for bioimaging and self-reporting drug delivery systems, particularly in the near-infrared (NIR) wavelength range 1000–1300 nm, where absorption of biological tissues and light scattering are favourable for signal penetration through deep tissues.2,3 On the other hand, doping with magnetic impurities4 in combination with the tuneable QD absorption could enable development of multifunctional labels for combined Magnetic Resonance Imaging (MRI) and fluorescence imaging.5 The complementarity of these imaging techniques, i.e. high spatial and temporal resolution of optical imaging and the non-invasive anatomical three dimensional (3D) imaging capability of MRI, could enable significant progress in medical diagnostics, for example, during surgical procedures, which require real-time image guidance and high resolution imaging.6
The advantages of colloidal semiconductor quantum dots for fluorescence imaging have been widely explored7,8 and particular interest has been recently focused on QDs with emission in the near-infrared wavelength range.9 On the other hand, the development of nanoparticles as MRI contrast agents has mainly directed to iron oxide particles,5,10 Mn-clusters11,12 and bi-metallic nanoparticles.13,14 However, these do not fluoresce. To achieve multifunctionality, different hybrid systems have been explored, including composite particles of QDs and iron oxides,5 and QDs with conjugated Gd(III)-chelates.15,16 These approaches significantly increase the size of the probes and thus the use of these nanostructures in medical imaging may be limited by inefficient transport through tissues and their clearance.17,18 Doping of colloidal QDs with transition metals (Mn, Fe, etc.) could offer an alternative strategy with minimal changes in size or morphology, as demonstrated for visible II–VI19,20 and I–III–VI21,22 QDs. In particular, Mn has been recently identified as a promising alternative to Gd in MRI.23 Mn-Based nanoparticles can have a relaxivity comparable to that of Gd(III) currently used in MRI as a contrast agent,24 but significantly lower intrinsic cytotoxicity.20 Although chelated Mn-complexes can also be employed in MRI, a Mn-doped QD would be less susceptible to chemical reactions and could be designed to combine within one structure magnetic properties with stable and tuneable absorption and emission, thus providing a promising route to multi-modal imaging. Furthermore, the nanoscale design of magnetic interactions within a QD has the potential to advance the use of nanoparticles in MRI and to open up new research directions in medical imaging.
Here we examine magnetic interactions in Mn-doped lead sulphide (PbS) semiconducting QDs capped with thioglycerols (TGL) and demonstrate that these NIR fluorescent QDs can be used as MRI labels (Fig. 1a and b). The capping molecules provide the nanocrystal with a thin shell, which enables the dispersion and solubilisation of the QDs in physiological solvents and allows free diffusion of the water molecules in close proximity to the QD surface, thus resulting in Mn-induced change of the magnetic resonance relaxation of hydrogen nuclei in surrounding water. We show that a Mn-doping of ∼5% results in a transverse relaxivity, r2 = 16 mM−1 s−1, comparable to that of super-paramagnetic iron oxide particles.27 However, we note that the use of iron oxide particles for cell transplantation tracking and intra-operative guidance is limited due to the hypointensity generated in MRI images being indistinguishable from small bleeds.26 Thus Mn-doped nanoparticles could be advantageous. Here we demonstrate the effect of our nanocrystals on neuronal cell viability and show that at non-toxic concentrations (<0.05 mg mL−1) the QDs, uptaken by neuronal cells, alter significantly the water relaxation properties thus providing contrast in MRI. Since these nanoparticles are also fluorescent with emission tuneable in the NIR,28,29 they have potential for applications in dual in vivo imaging.
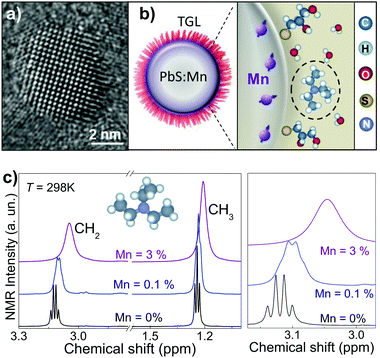
Colloidal Mn-doped PbS nanocrystals are synthesised in aqueous solution with Mn-content up to 7%. A mixture of 1-thioglycerol and 2,3-dimercapto-1-propanol is used to stabilise the nanocrystals and to make them water soluble. Triethylamine (Et3N) is used to control the pH of the QD solution. Mn2+ is incorporated as substitutional impurity into the Pb-sublattice thus forming alloyed (PbMn)S nanocrystals with room temperature photoluminescence (PL) emission tuneable by Mn-content in the range 850–1200 nm.25 Transmission electron microscopy (TEM) and high-resolution (HR) TEM images indicate that the QDs have average diameter d = 4.5 ± 1.2 nm, independent of Mn content, and confirm that alloyed nanocrystals retain high crystallinity (Fig. 1 and ESI,† Fig. S1).
NMR provides a powerful technique for determining the effect of paramagnetic impurities on proton spin relaxation time constants (i.e. T1 and T2) as well as a method for structural characterization. Here we use NMR spectroscopy to probe the interaction between the paramagnetic Mn2+ ions encapsulated into the QDs and the protons in surrounding molecules. In particular, we focus on the relaxation properties of protons of the –CH3 and –CH2 groups of Et3N molecules present in the solvent. The NMR spectra of PbS and (PbMn)S QDs recorded at T = 298 K (Fig. 1c) show two intense and resolved peaks. By comparing these spectra to that of Et3N in water (not shown), we assign the quartet at 3.12 ppm and the triplet at 1.24 ppm to the –CH2 and –CH3 groups, respectively.30 We attribute the multiplet splitting to the spin–spin scalar coupling J = 7.7 Hz for both –CH3 and –CH2, which is unchanged with respect to those of the pure Et3N sample. However, for PbS QD solutions we observe a significant down-field chemical shift of the resonance peaks of Et3N by 0.71 ppm and 0.35 ppm for –CH3 and –CH2 groups, respectively. We note that the chemical shift and J-couplings are independent of the proton concentration (i.e. H2O/D2O ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120) and pH of the solution. Incorporation of Mn in the QDs leads also to an up-field shift of 0.06 ppm for CH2 and of 0.035 ppm for CH3 groups, and broadening of the resonance lines. For a Mn-content larger than 0.1%, the multiplet structures cannot be resolved. The broadening suggests that the local magnetic moment of the (PbMn)S QDs induces paramagnetic relaxation on the protons of the Et3N molecules.
120) and pH of the solution. Incorporation of Mn in the QDs leads also to an up-field shift of 0.06 ppm for CH2 and of 0.035 ppm for CH3 groups, and broadening of the resonance lines. For a Mn-content larger than 0.1%, the multiplet structures cannot be resolved. The broadening suggests that the local magnetic moment of the (PbMn)S QDs induces paramagnetic relaxation on the protons of the Et3N molecules.
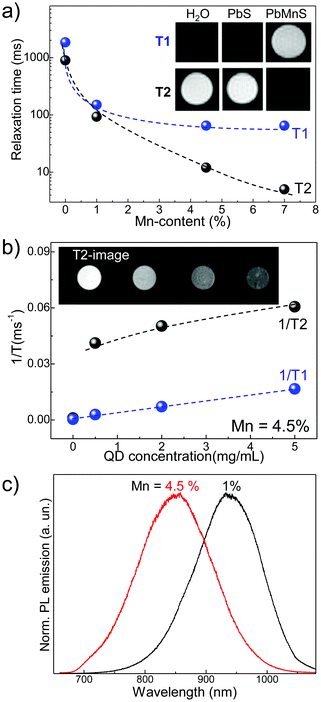
Analysis of the proton relaxation times in –CH2 and –CH3 groups of Et3N show a two orders of magnitude decrease from T1 = 2.40 ± 0.09 s for –CH2 and T1 = 2.66 ± 0.04 s for –CH3 groups in the PbS QD solution to 0.024 ± 0.003 s and 0.027 ± 0.002 s, respectively, for (PbMn)S with Mn = 3%. The length of the capping molecules used to terminate the growth of the nanocrystals is <0.5 nm and allows free diffusion of Et3N molecules to the QD surface resulting in a significant decrease of the relaxation times via paramagnetic relaxation effects. Similar effects are observed for the relaxation properties of water proton spins in MRI. As shown in Fig. 2a, our MRI studies show that T1 and T2 relaxation times are significantly decreased by an increasing Mn-content. For solutions with 7% Mn and QD concentration of 5 mg mL−1, we observe a decrease of T1 and T2 by one and two orders of magnitude, respectively, compared to identical control samples with 0% Mn. This increase of relaxation rates by ∼100-times is comparable to the change estimated in our NMR studies. We note that in undoped PbS QD solutions the relaxation rate of the water protons is not noticeably affected by the presence of the nanocrystals. As shown in the inset of Fig. 2a, the Mn-induced change of T1 and T2 provides significant contrast in MRI images of QD solutions.
We envisage that Mn-ions in closer proximity to the QD surface affect predominantly the relaxation of the surrounding water protons. By assuming a uniform distribution of Mn throughout a spherical nanocrystal, we estimate that the number of Mn-ions within a distance of 0.5 nm from the QD surface increases from ∼25 for Mn = 1% to ∼150 for Mn = 7%. On the other hand, the broadening of the NMR lines and the non-linear decrease of T1 and T2 with increasing Mn-content indicate much stronger effects at low Mn content (Mn < 1%), thus suggesting the preferential location of Mn close to the periphery of the nanocrystal.31 Self-purification mechanisms4 can lead to impurity out-diffusion resulting in greater impurity content in the proximity of the QD surface compared to the centre.32 The greater Mn-dependent reduction of T1 and T2 times at low Mn compared to higher Mn content indicates a saturation of the concentration of Mn-ions close to the QD surface.
Representative T1 and T2 times of serial dilution of (PbMn)S QDs with 4.5% and corresponding T2-weighted images are shown in Fig. 2b. To facilitate the comparison with other works, we assess the relaxivity per Mn concentration: at room temperature and magnetic field B = 7 T, we find r1 = 5 mM−1 s−1 and r2 = 16 mM−1 s−1, for T1 and T2, respectively. The paramagnetism of the QDs, water accessibility and rotational dynamics are likely causes for the more pronounced T2 effect compared to T1 in these QDs. To assess the effect of environment viscosity, and thus water diffusion, on the QD-induced changes of relaxivity, we performed the MRI studies of QDs dispersed in 0.5% agarose and found no noticeable differences in the values of T1 and T2 compared to those in water. This suggests that similar effects should be observed in cells and/or tissues. Our investigations demonstrate relaxivity values that are similar or greater than those reported for Mn-doped II–VI QDs.19,20 Remarkably, the relaxivity values of r2 are similar to those of Fe-based superparamagnetic nanoparticles (SPIONs)26 and only 10-times lower compared to commercial Fe-based contrast agents.33 Since our Mn-doped QDs are significantly smaller (d ∼ 5 nm) compared to clinically approved Fe-based particles (d > 100 nm),33 the greater cellular uptake combined with the large relaxation rates could provide better contrast in MRI. Also, following the incorporation of Mn, the QDs remain optically active in the NIR wavelength range, as shown in Fig. 2c for Mn = 1 and 4.5%.
Imaging of neural cells is of great importance for assessing cell transplant therapies34 and Mn-based contrast agents have been considered of great promise.11,35,36 One of the main concerns in applications of the QDs is their penetration through the blood–brain barrier, which could be controlled by functionalization of the nanocrystals with cell penetration peptides.37 To investigate the potential of our (PbMn)S QDs for neuronal imaging, we have exposed the N2a mouse neuroblastoma cells to QDs with Mn content of 4.5% for 24 hours. Our TEM images reveal localized QD clusters in the cytoplasm indicating that the cellular uptake occurs via endocytosis.38,39 The cellular uptake is sufficient to allow photoluminescence/absorption of the QDs to be detected in cells and cell pellets. For MRI, the cell pellets of equal volume in Eppendorf tubes were positioned at the centre of a 7 T magnet. T2-Weighted MRI images of cell pellets (Fig. 3) reveal a QD-induced contrast for QD treatment concentrations of 0.005 mg mL−1 and 0.01 mg mL−1. The 3-fold change in MRI intensity for the cells exposed to (PbMn)S QDs compared to the control sample is easily discernible by eye and comparable to the contrast achieved with SPIONs23 and in Mn-enhanced MRI.11,36
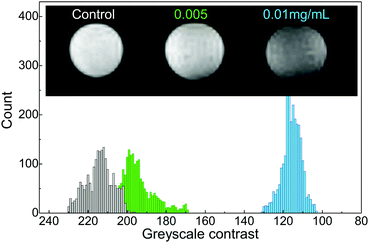 | ||
| Fig. 3 T 2-Weighted image of neuronal cell pellets following 24 h exposure to (PbMn)S QDs (Mn = 5%) and a corresponding histogram of the detected MRI signal. | ||
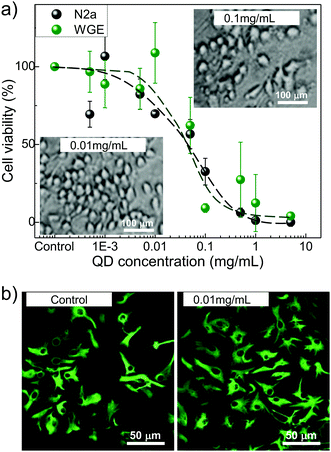
We note that the QD concentrations used in MRI are non-toxic to the cell. We compare the cytotoxicity of (PbMn)S in two representative cell lines: N2a and WGE. The N2a is an immortal neural cell line derived from mouse neuroblastoma tumours that can be differentiated into neurones. The primary WGE cells were dissected from developing mouse embryos at E14 and have the potential to become neurones or glial cells of the adult striatum. For MTT analysis to test neuronal viability in vitro, the N2a and WGE cells were treated with (PbMn)S QDs for up to 72 h (Fig. 4a).
Additionally, primary cells were assessed over 7 days following exposure to QDs. The highest dose which did not induce significant cell death at any time point (24, 48 and 72 hours and 7 days) in the primary cell cultures was determined to be 0.01 mg mL−1, and there was a trend towards N2a cells being less resilient to the effects of the QDs. The growth inhibition values for N2a and WGE cells (GI50= 0.05 mg mL−1) are lower compared to fibroblast cells,35 indicating higher sensitivity of neuronal cells to QD induced toxicity. Optical microscopy assessment of N2a cells revealed reduced cell density and loss of membrane integrity at QD treatment concentrations above 0.1 mg mL−1, indicative of cell death (inset in Fig. 4a). Following 7 day treatment with 0.01 mg mL−1 (PbMn)S QDs (Fig. 4b) GFAP immunocytochemistry analysis revealed no alterations to the differentiation and morphology of cells. However, at treatment concentration of >0.05 mg mL−1, cell death occurred and surviving GFAP neurones exhibited altered morphology. There is no noticeable release of Mn-ions from the QD since the QD induced toxicity levels are comparable to those of PbS QDs and are independent of the Mn-content. Recent studies raised concerns about possible ROS-induced cell damage (e.g. DNA damage) following exposure to nanoparticles.40,41 We note that at concentrations below GI50 values we do not observe any QD-induced changes to cell viability and proliferation as probed by MTT assay, cell cycle and annexin V studies, and ROS.28,38 Our previous acute toxicity studies have demonstrated that PbS-based QDs do not accumulate in the organs, are well tolerated by mice, and their NIR photoluminescence can be imaged in vivo.34
Conclusions
In conclusion, we have demonstrated that Mn-doped PbS QDs can be used as biocompatible MRI probes. By encapsulating the nanocrystals with a thin layer of capping molecules, we enable the free diffusion of water molecules to the QD surface resulting in a decrease of proton relaxation times and contrast in MRI. At the concentrations where these QDs do not exhibit cellular toxicity, the T2-weighted images reveal a 3-fold change of MRI intensity. The combination of paramagnetism and fluorescence emission in the near-infrared wavelength range of low absorption of biological tissues makes these QDs promising candidates for dual medical imaging. Presently, specific targeting of these QDs is being explored and combined in vivo mice imaging is being developed in conjunction with extension of the detection range of current fluorescence live animal imaging systems to the NIR.Experimental section
Morphological, optical and NMR studies
For the transmission electron microscopy (TEM) study, the nanocrystals were deposited on a graphene-oxide coated grid and TEM images were recorded on a JEOL1200EX microscope operating at 120 kV.Optical properties of quantum dots were measured in solution and in agarose gel pellets. The optical excitation was provided by the 532 nm line of a solid state laser and an excitation power in the range P = 10–100 W m−2. The luminescence was dispersed by a 150 g mm−1 grating and detected by either a nitrogen-cooled (InGa)As array photodiode or a charge-coupled device (CCD). We note that aqueous solutions of PbS:Mn are stored at T = 4 °C under nitrogen atmosphere and are stable over a period of at least 3 month. The colloidal stability of aqueous nanoparticles can be altered by oxidation of the surface and/or removal of the capping ligands and care should be taken when subjecting QDs to high temperatures (T > 50 °C) and/or low pH environment. The Nuclear Magnetic Resonance (NMR) data were collected at 600 MHz on a Bruker Avance III spectrometer at 298 K on QDs dispersed in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O (1
D2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Solvent suppression was achieved using excitation sculpting, where required. T1 experiments were conducted with an inversion recovery sequence: π–delay (T)–π/2–free induction decay. Data were acquired as a pseudo 2D and the relaxation delay was set at >5 × T1 to facilitate complete recovery between transients. Data were phased and baseline corrected prior to integration using TOPSPIN 3 software.
9). Solvent suppression was achieved using excitation sculpting, where required. T1 experiments were conducted with an inversion recovery sequence: π–delay (T)–π/2–free induction decay. Data were acquired as a pseudo 2D and the relaxation delay was set at >5 × T1 to facilitate complete recovery between transients. Data were phased and baseline corrected prior to integration using TOPSPIN 3 software.
Magnetic resonance imaging (MRI)
All MRI experiments were performed on a horizontal Bruker Biospec 7 T Avance III scanner (Bruker Biospin MRI GmbH, Ettlingen, Germany) with a 7 cm transmit/receive volume coil. QD solutions were extensively dialysed to remove the residual free Mn-ions. 1 mL of samples in Eppendorf tubes were positioned in a polystyrene holder. Relaxometry measurements were performed by taking a gradient echo localiser scan to establish central positioning of the samples in the magnet, followed by a Rapid Acquisition with Relaxation Enhancement Variable Time of Repetition (RARE-VTR) fast spin echo T1/T2 scan. Scanner software (Paravision 5.1) was used to determine a 0.15 cm2 region of interest for each sample and to obtain relaxation values. A series of eight echo times (TE from 6.5 ms to 240 ms) and inversion times (TI from 30 ms to 13 s) were optimised, and T1 and T2 relaxation curves were plotted. The following parameters were used for all relaxation measurements: matrix size = 128 × 128, field of view (FOV) 80 mm/70 mm × 50 mm, slice thickness 1.5 mm and acquisition time τaq ∼ 11 min.MTT and immunofluorescence assays
The cells were seeded into 96-well plates at a density of 6 × 103 per well and allowed 24 hours to adhere before treatment with serially diluted QD solutions (from 5 mg mL−1 to 0.5 μg mL−1) for up to 72 h. Control wells received the vehicle alone. Following exposure, MTT was added to each well at a final concentration of 5 mg mL−1 and incubated at 37 °C for 30 min. Supernatants were aspirated and cellular formazan solubilised by the addition of DMSO and absorbance was read at 550 nm. Primary striatal cells from mouse embryos (WGE) were seeded at a density 5 × 104 per well and were exposed to QDs (0, 0.01, 0.05 and 0.1 mg mL−1) for 7 days. Then the cells were fixed with 4% paraformaldehyde for 15 minutes, washed several times in PBS, permeabilised for 10 minutes (PBS with 0.25% Triton X-100) and blocked with 10% goat serum, 0.25% Triton X-100 and 0.3 M glycine in PBS for 1 hour. Primary antibodies were diluted as follows: NeuN (to visualise neuronal cell nuclei, monoclonal; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) and GFAP (to visualise glial cells, polyclonal; 1
2000) and GFAP (to visualise glial cells, polyclonal; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) in 1% BSA, 0.3 M glycine, 0.25% Triton X-100 in PBS and were incubated overnight at 4 °C. The cells were washed extensively with PBS and then incubated in the dark with the secondary antibody (1
1000) in 1% BSA, 0.3 M glycine, 0.25% Triton X-100 in PBS and were incubated overnight at 4 °C. The cells were washed extensively with PBS and then incubated in the dark with the secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) in 1% BSA, 0.25% Triton X-100 and 0.3 M glycine in PBS for 1 hour. After further three PBS washes, the cells were counter stained with DAPI (1
500) in 1% BSA, 0.25% Triton X-100 and 0.3 M glycine in PBS for 1 hour. After further three PBS washes, the cells were counter stained with DAPI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), washed in distilled water. The cover slip was applied using DABCO mounting medium before being viewed under a Nikon microscope.
1000), washed in distilled water. The cover slip was applied using DABCO mounting medium before being viewed under a Nikon microscope.
Acknowledgements
The work was supported by funding from The Leverhulme Trust (Grant No. RPG-2013-242), NC3Rs/EPSRC (Grant No. NC/L001861/1), the EPSRC Impact Acceleration Account (EP/K503800/1) and The University of Nottingham. We acknowledge useful discussions and assistance with TEM and confocal imaging from Dr D. McLean and Dr M. W. Fay.Notes and references
- R. Saran and R. J. Curry, Nat. Photonics, 2016, 10, 81–92 CrossRef CAS.
- G. Hong, S. Diao, J. Chang, A. L. Antaris, C. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo and H. Dai, Nat. Photonics, 2014, 8, 723 CrossRef CAS PubMed.
- R. Koole, W. J. M. Mulder, M. M. van Schooneveld, G. J. Strijkers, A. Meijerink and K. Nicolay, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 475 CrossRef CAS PubMed.
- D. J. Norris, A. L. Efros and S. C. Erwin, Science, 2008, 28, 1776 CrossRef PubMed.
- L. Jing, K. Ding, S. V. Kershaw, I. M. Kempson, A. L. Rogach and M. Gao, Adv. Mater., 2014, 26, 6367 CrossRef CAS PubMed.
- J. Jin, Z. Xu, Y. Zhang, Y. J. Gu, M. H. Lam and W. T. Wong, Adv. Healthcare Mater., 2013, 2(11), 1501 CrossRef CAS PubMed.
- M. X. Zhao and E. Z. Zeng, Nanoscale Res. Lett., 2015, 10, 171 CrossRef PubMed.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS PubMed.
- T. Jin and Y. Imamura, ECS J. Solid State Sci. Technol., 2016, 5, R3138–R3145 CrossRef CAS.
- L. Zhang, Y. Wang, Y. Tang, Z. Jiao, C. Xie, H. Zhang, P. Gu, X. Wei, G.-Y. Yang, H. Gu and C. Zhang, Nanoscale, 2013, 5, 4506 RSC.
- J. Moraes Malheiros, F. Fernandes Paiva, B. Monteiro Longo, C. Hamani and L. Covolan, Frontiers in Neurology, 2015, 6(161), 1–10 Search PubMed.
- M. Kueny-Stotz, A. Garofalo and D. Felder-Flesch, Eur. J. Inorg. Chem., 2012, 1987 CrossRef CAS.
- P. C. Naha, A. A. Zaki, E. Hecht, M. Chorny, P. Chhour, E. Blankemeyer, D. M. Yates, W. R. Witschey, H. Litt, A. Tsourkas and D. P. Cormode, J. Mater. Chem. B, 2014, 2, 8239 RSC.
- P. C. Naha, K. C. Lau, J. C. Hsu, M. Hajfathalian, S. Mian, P. Chhour, L. Uppuluri, E. S. McDonald, A. D. A. Maidment and D. P. Cormode, Nanoscale, 2016, 8, 13740 RSC.
- Q. An, J. Liu, M. Yu, J. Wan, D. Li, C. Wang, C. Chen and J. Guo, Small, 2015, 11, 5675–5686 CrossRef CAS PubMed.
- C.-L. Huang, C.-C. Huang, F.-D. Mai, C.-L. Yen, S.-H. Tzing, H.-T. Hsieh, Y.-C. Ling and J.-Y. Chang, J. Mater. Chem. B, 2015, 3, 651 RSC.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3(5), 703 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. I. Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165 CrossRef CAS PubMed.
- V. K. Sharma, S. Gokyar, Y. Kelestemur, T. Erdem, E. Unal and H. V. Demir, Small, 2014, 10(23), 4961 CrossRef CAS PubMed.
- S. Wang, B. R. Jarrett, S. M. Kauzlarich and A. Y. Louie, J. Am. Chem. Soc., 2007, 129, 3848 CrossRef CAS PubMed.
- K. Ding, L. Jing, C. Liu, Y. Hou and M. Gao, Biomaterials, 2014, 35, 1608–1617 CrossRef CAS PubMed.
- G. Sitbon, S. Bouccara, M. Tasso, A. Francois, L. Bezdetnaya, F. Marchal, M. Beaumonte and T. Pons, Nanoscale, 2014, 6, 9264–9272 RSC.
- E. M. Gale, I. P. Atanasova, F. Blasi, I. Ay and P. Caravan, J. Am. Chem. Soc., 2015, 137, 15548 CrossRef CAS PubMed.
- J. G. Penfield and R. F. Reilly Jr., Nat. Clin. Pract. Nephrol., 2007, 3, 654–668 Search PubMed.
- M. Corti, A. Lascialfari, E. Micotti, A. Castellano, M. Donativi, A. Quarta, P. D. Cozzoli, L. Manna, T. Pellegrino and C. Sangregorio, J. Magn. Magn. Mater., 2008, 320, e320 CrossRef CAS.
- E. Tarulli, J. D. Chaudhuri, V. Gretka, A. Hoyles, C. M. Morshead and G. J. Stanisz, J. Magn. Reson. Imaging, 2013, 37(6), 1409 CrossRef PubMed.
- J. Xiao, X. M. Tian, C. Yang, P. Liu, N. Q. Luo, Y. Liang, H. B. Li, D. H. Chen, C. X. Wang, L. Li and G. W. Yang, Sci. Rep., 2013, 3, 3424 CAS.
- L. Turyanska, F. Moro, A. N. Knott, M. W. Fay, T. D. Bradshaw and A. Patanè, Part. Part. Syst. Charact., 2013, 30, 945 CrossRef CAS.
- L. Turyanska, R. J. A. Hill, O. Makarovsky, F. Moro, A. N. Knott, O. J. Larkin, A. Patanè, A. Meaney, P. C. M. Christianen, M. W. Fay and R. J. Curry, Nanoscale, 2014, 6(15), 8919 RSC.
- SDBSWeb, http://sdbs.db.aist.go.jp, National Institute of Advanced Industrial Science and Technology.
- F. Moro, L. Turyanska, J. Wilman, H. E. L. Williams, A. J. Fielding and A. Patanè, Nano Lett., 2016, 16, 6343–6348 CrossRef CAS PubMed.
- F. Moro, L. Turyanska, J. Granwehr and A. Patanè, Phys. Rev. B, 2014, 90, 205428 CrossRef.
- Y.-X. J. Wang, Quant. Imaging Med. Surg., 2001, 1(1), 35 Search PubMed.
- M. Modo, J. S. Beech, T. J. Meade, S. C. R. Williams and J. Price, NeuroImage, 2009, 47, T133 CrossRef PubMed.
- A. C. Silva, J. H. Lee, I. Aoki and A. P. Koretsky, NMR Biomed., 2004, 17, 532 CrossRef CAS PubMed.
- R. Pautler, A. C. Silva and A. P. Koretsky, Magn. Reson. Med., 1998, 40, 740 CrossRef CAS PubMed.
- S. Santra, H. Yang, P. H. Holloway, J. T. Stanley and R. A. Mericle, J. Am. Chem. Soc., 2005, 127, 1656 CrossRef CAS PubMed.
- T. D. Bradshaw, M. Junor, A. Patanè, P. Clarke, N. R. Thomas, M. Li, S. Mann and L. Turyanska, J. Mater. Chem. B, 2013, 1, 6254 RSC.
- L. Turyanska, T. D. Bradshaw, J. Sharpe, M. Li, S. Mann, N. R. Thomas and A. Patanè, Small, 2009, 5(15), 1738 CrossRef CAS PubMed.
- K. Bhattacharya, P. C. Naha, I. Naydenova, S. Mintova and H. J. Byrne, Toxicol. Lett., 2012, 215, 151 CrossRef CAS PubMed.
- P. C. Naha, M. Davoren, F. M. Lyng and H. I. Byrne, Toxicol. Appl. Pharmacol., 2010, 246, 91 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tb02574a |
| This journal is © The Royal Society of Chemistry 2016 |