Hollow mesoporous hetero-NiCo2S4/Co9S8 submicro-spindles: unusual formation and excellent pseudocapacitance towards hybrid supercapacitors†
Linrui
Hou
a,
Yaoyao
Shi
a,
Siqi
Zhu
a,
Muhammad
Rehan
a,
Gang
Pang
ab,
Xiaogang
Zhang
b and
Changzhou
Yuan
*a
aSchool of Materials Science & Engineering, Anhui University of Technology, Ma'anshan, 243002, P. R. China. E-mail: ayuancz@163.com
bCollege of Material Science & Engineering, Nanjing University of Aeronautics & Astronautics, Nanjing, 210016, P. R. China
First published on 5th October 2016
Abstract
Hierarchical hollow porous architectures with intriguing hetero-interfaces are currently of particular interest in emerging energy-related fields. In this investigation, we report a smart template-free methodology to purposefully fabricate high-quality uniform hollow hetero-NiCo2S4/Co9S8 (NCCS) submicro-spindles with well-dispersed hetero-nanodomains at the nanoscale. High-yield hollow mesocrystal nickel cobalt carbonate spindles are first solvothermally synthesized as the intermediate, and a subsequent shape-preserving conversion into hetero-NCCS submicro-spindles via a hydrothermal anion-exchange reaction occurs. The underlying template-free formation mechanism of the hollow structures is tentatively proposed. When evaluated as a promising electrode for supercapacitors, the resultant hollow mesoporous hetero-NCCS electrode with a mass loading of 5 mg cm−2 delivers a good pseudocapacitance of ∼749 F g−1 at a current rate of 4 A g−1, and holds at approximately 620 F g−1 at 15 A g−1 as a result of intrinsic synergetic contributions from structural/compositional/componental merits. Furthermore, an asymmetric device based on hollow mesoporous hetero-NCCS achieves an encouraging energy density of around 33.5 W h kg−1 at a power density of 150 W kg−1, and exceptional cycling behavior with capacitance degradation of ∼0.007% per cycle over 5000 consecutive cycles at 5 A g−1. Comprehensive investigations unambiguously highlight that the unique hollow mesoporous hetero-NCCS submicro-spindles would be a powerful electrode platform for advanced next-generation supercapacitors.
1. Introduction
Nowadays, the accelerated exhaustion of traditional fossil fuels and serious ecological pollution greatly spur tremendous development of various renewable and environmentally benign energy conversion/storage systems.1,2 Electrochemical supercapacitors (ESCs), as a rising star with inherent unusual properties, stand out from other electrochemical devices, and have gradually received worldwide attention because of their high charging/discharging rates, long-term lifespan, environmental friendliness, high reliability and so forth. They, bridging the second rechargeable batteries and physical dielectric capacitors, have been utilized extensively in solar power systems to avoid power fluctuations, and as strong power sources in electric vehicles (EVs), hybrid/plug-in EVs, portable electronic devices, space/military devices, and large industrial equipment. In general, ESCs can be classified into two categories according to their intrinsic energy-storage mechanisms.3–5 One is the electric double-layer capacitors (EDLCs), which commonly build up electrical charge at the electrode–electrolyte surfaces/interfaces, as described by the well-established Gouy–Chapman–Stern–Grahame model, and the other is pseudocapacitors, which originate from typical faradaic redox reactions taking place at the interfaces under certain potentials.3,4 Of especial note is that the latter exhibit larger specific capacitances (SCs) than the former, thanks to their typical pseudocapacitive mechanism.3–5In this regard, various attempts and considerable endeavors have thus been devoted to investigating pseudocapacitive electrodes with excellent electrochemical performance. With their unique charge-storage mechanism in mind, several desirable features are necessary for advanced pseudocapacitive electrodes, including large electroactive surface area, high electrochemical activity and good electrical conductivity,1,3,5 which can synchronously guarantee ions/electrons to rapidly contact sufficient electroactive sites for efficient electrochemical energy storage via fast reversible faradaic redox reactions. It is therefore both significant and imperative to explore suitable functional materials, and further finely tailor and engineer their micro-structures and specific components to meet the aforementioned prerequisites.
Bimetallic (Ni–Co) sulfide (NiCo2S4) with a band-gap energy (Eg) of ∼2.5 eV,6 as a low-cost competitive electrode candidate, has received extensive attention in the ESCs field since the first report in 2013,7 owing to favorable synergetic effects from both Ni and Co species with multiple valence transitions for reversible faradaic reactions in aqueous KOH solution.8,9 More excitingly, ternary NiCo2S4 is endowed with exceptional electrochemical activity, higher than any single-component sulfide and even its mixed oxide counterpart (NiCo2O4),10 benefiting from its very high electronic conductivity (∼100 times higher than that of NiCo2O4, and approximately four orders of magnitude higher than those for conventional transitional metal oxides).11,12 In recent years, cobalt sulfide (Co9S8, Eg of ∼3.2 eV (ref. 13)), as another emerging alternative electrode for high-performance ESCs, has exhibited more remarkable electrochemical capacitances as well owing to its high electro-activity and good electronic conductivity.13–16
To further improve the electric conductivity of the pseudocapacitive electrode itself, one exciting avenue is opened by constructing novel hetero-structures coupling well-dispersed nano-domains with different Eg at the nanoscale.17–22 As a result, an enhanced internal electric field is induced at the hetero-phase interfaces, and the corresponding built-in charge transfer driving force would ameliorate the surface reaction kinetics, and facilitate the interfacial electron transport at hetero-interfaces over the whole electrochemical reactions.19 Ultrafast charge transfer and electron mobility can be successfully achieved in several innovative and impressive cases, for instance, MoS2/WS2,17 CdO/SnTe,18 SnS/SnO2,19 ZnO/ZnFe2O4,20 TiO2(B)–anatase,21 Ni7S6/Co3S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 hetero-structures, and so on. Furthermore, unique hollow micro- and/or submicro-structures can be constructed with convenient ion diffusion/transport pathways, high surface-to-bulk ratio, advantageous electroactive surfaces/interfaces for fast ion absorption/desorption and faradaic reactions, giving rise to large high-current-rate SCs.5,8,9,22,23 More strikingly, unique micro-/submicro-architectures would additionally result in high tap density and desirable mechanical properties to some extent, which isimportant for practical applications,1,5,24 nevertheless, there is still an urgent need for low-cost high-yield fabrication of hollow architectures, particularly via template-free strategies, avoiding the conventional utilization of soft/hard templates, and simplifying tedious synthetic procedures.23
22 hetero-structures, and so on. Furthermore, unique hollow micro- and/or submicro-structures can be constructed with convenient ion diffusion/transport pathways, high surface-to-bulk ratio, advantageous electroactive surfaces/interfaces for fast ion absorption/desorption and faradaic reactions, giving rise to large high-current-rate SCs.5,8,9,22,23 More strikingly, unique micro-/submicro-architectures would additionally result in high tap density and desirable mechanical properties to some extent, which isimportant for practical applications,1,5,24 nevertheless, there is still an urgent need for low-cost high-yield fabrication of hollow architectures, particularly via template-free strategies, avoiding the conventional utilization of soft/hard templates, and simplifying tedious synthetic procedures.23
Herein, it should be pointed out that each proposed strategy possesses its own unique advantages, as discussed above, however limited progress in the overall electrochemical capacitance would be delivered if each design was utilized alone. From this point of view, a smart marriage of electroactive NiCo2S4 with hollow micro-/submicro-structures and hetero-interfaces in principle would offer a more competitive electrode candidate for high-performance ESCs. However, it remains a major challenge to develop simple yet scalable protocols for fine manipulation and high-yield fabrication of integrated electrodes with outstanding supercapacitance for ESCs. With the comprehensive considerations and motivations above, we devised in the present work a template-free synthetic methodology for the large-scale synthesis of well-defined hollow mesoporous hetero-NiCo2S4/Co9S8 (hereafter denoted as NCCS) submicro-spindles with well-dispersed NiCo2S4/Co9S8 hetero-interfaces at the nanoscale. When tested as a cost-efficient pseudocapacitive electrode for ESCs, the hollow mesoporous hetero-NCCS submicro-spindles with a mass loading of 5 mg cm−2 exhibited good supercapacitances at high rates. Moreover, an activated carbon (AC)//hollow hetero-NCCS asymmetric supercapacitor was assembled with 6 M KOH as the aqueous electrolyte, and achieved a high specific energy density (SED) of ∼33.5 W h kg−1 in terms of electroactive materials in two electrodes, and long-term cycling duration with a SC degradation of ∼0.006% per cycle after thousands of consecutive charge–discharge cycles.
2. Experimental section
2.1. Synthesis of the hollow NCCO intermediate
The chemicals here were all of analytical grade, and used directly as purchased without any purification. In total, 0.5 mmol of Ni(CH3COO)2·4H2O and 1.0 mmol of Co(CH3COO)2·4H2O were dissolved well into 40 mL of ethylene glycol (EG). 15 mmol of NH4HCO3 was then added into the above solution under stirring at room temperature (RT). Afterwards, the clear solution was transferred to a Teflon-lined autoclave (50 mL), and then kept at 200 °C for 20 h. After being separated, washed and dried at 80 °C, the product was obtained, and the as-fabricated Ni/Co-based carbonates were denoted as NCCO for convenience. For comparison, different amounts of NH4HCO3, such as 10, 20 and 30 mmol, were applied, and the corresponding products were marked as NCCO-10, NCCO-20 and NCCO-30, respectively. Furthermore, the time-dependent evolution of the NCCO samples with different hydrothermal durations (0.5 h, 1.5 h, 5 h, 8 h, 10 h and 25 h) was also investigated, and the as-obtained samples were labeled as NCCO-0.5 h, NCCO-1.5 h, NCCO-5 h, NCCO-8 h, NCCO-10 h and NCCO-25 h, respectively.2.2. Synthesis of hollow mesoporous hetero-NCCS submicro-spindles
Typically, 0.2 g of the NCCO sample was put into 40 mL of aqueous solution with 1.2 g of Na2S·9H2O. After mixing by ultrasonication, the mixture was transferred into a 50 mL Teflon-lined autoclave and held at 160 °C for 15 h in an electric oven, resulting in a black sample of hollow mesoporous hetero-NCCS submicro-spindles.2.3. Sample characterization
The products were analyzed by powder X-ray diffraction (XRD) on a multipurpose D8-Advance XRD system from Bruker with a Cu Kα source (λ = 0.154056 nm) at a scanning speed of 2° min−1 over a 2θ range of 10–80°. Morphologies and structures were observed by field-emission scanning electron microscopy (FESEM, JEOL-6300F, 15 kV), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and selected area electron diffraction (SAED) (JEOL JEM 2100 system operating at 200 kV). N2 adsorption/desorption isotherms were determined by Brunauer–Emmett–Teller (BET) measurement using an ASAP-2020 surface area analyzer at the temperature of liquid nitrogen. The pore size distribution (PSD) was obtained from the adsorption branch of the pore size distribution curve obtained via the Barrett–Joyner–Halenda (BJH) method. X-ray photoelectron spectroscopy (XPS) measurements were performed on a PHi5000 X-ray photoelectron spectrometer with an Al Kα excitation source (1486.6 eV), the spectra were fitted with the XPSPEAK41 program. The compositional analysis was completed using an X-ray fluorescence spectrometer (XRFS, ARL Advant'X 3600).2.4. Electrochemical measurements
The electrochemical performance of hollow hetero-NCCS submicro-spindles was investigated comprehensively both with a three-electrode configuration and a two-electrode hybrid device. The working electrode in the 3-electrode setup was fabricated with hollow hetero-NCCS, conductive acetylene black (AB) and polytetrafluoroethylene (PTFE) with a weight proportion of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. De-ionized (DI) water was added to make the mixture more homogeneous, which was then pressed (15 MPa) onto a fresh nickel foam (1 cm2). The average mass loading of the electroactive material was 5 mg cm−2 for each electrode. Electrochemical properties were evaluated in the three-electrode system by cyclic voltammetry (CV), chronopotentiometry (CP) and electrochemical impedance spectroscopy (EIS) measurements with an IVIUM electrochemical workstation (the Netherlands) at RT. Cycling performance was performed with a CT2001D tester (Wuhan, China). A Pt plate (1 cm2) and saturated calomel electrode (SCE) were used here as the counter and reference electrodes, respectively. An aqueous solution of 6 M KOH was chosen as the electrolyte for all the electrochemical measurements. The EIS capacitance (SCEIS) of the electrode can be calculated from the imaginary component of the impedance by using the following equation:
1. De-ionized (DI) water was added to make the mixture more homogeneous, which was then pressed (15 MPa) onto a fresh nickel foam (1 cm2). The average mass loading of the electroactive material was 5 mg cm−2 for each electrode. Electrochemical properties were evaluated in the three-electrode system by cyclic voltammetry (CV), chronopotentiometry (CP) and electrochemical impedance spectroscopy (EIS) measurements with an IVIUM electrochemical workstation (the Netherlands) at RT. Cycling performance was performed with a CT2001D tester (Wuhan, China). A Pt plate (1 cm2) and saturated calomel electrode (SCE) were used here as the counter and reference electrodes, respectively. An aqueous solution of 6 M KOH was chosen as the electrolyte for all the electrochemical measurements. The EIS capacitance (SCEIS) of the electrode can be calculated from the imaginary component of the impedance by using the following equation: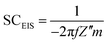 | (1) |
The asymmetric configuration was constructed by using the hollow hetero-NCCS spindles and AC (∼227 F g−1 at 5 A g−1)22 as positive and negative electrodes face to face in 6 M KOH solution. According to the charge-balance principle (Q+ = Q−), where Q+ and Q− represent the charges stored in the positive and negative electrodes, respectively, the specific mass ratio of the AC to the hollow hetero-NCCS was thus designed as 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in such an asymmetric device. The total mass of electroactive materials in the hybrid was 21 mg. The specific capacitances (SCs) of the electrode and/or hybrid cell were calculated from the CP plots according to eqn (2):
5 in such an asymmetric device. The total mass of electroactive materials in the hybrid was 21 mg. The specific capacitances (SCs) of the electrode and/or hybrid cell were calculated from the CP plots according to eqn (2):
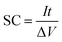 | (2) |
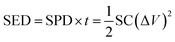 | (3) |
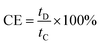 | (4) |
3. Results and discussion
3.1. Physicochemical, textural and structural characterization
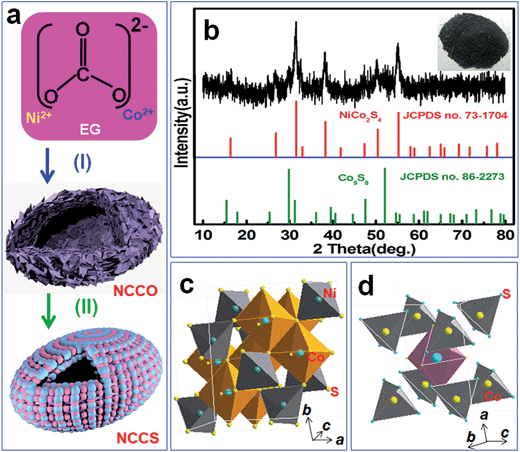
The schematic depiction in Fig. 1a demonstrates our design concept for the straightforward synthesis of hollow mesoporous hetero-NCCS submicro-spindles. More specifically, the template-free strategy developed in this contribution involves the facile solvothermal fabrication of a hollow NCCO precursor (step I), with a subsequent anion-exchange conversion process (step II) to prepare hollow hetero-NCCS submicro-spindles. During the initial solvothermal process in EG solution, the Ni2+/Co2+ ions react with the released CO32− ion from the decomposition of NH4HCO3,25 resulting in a gray NCCO intermediate (inset in Fig. S1, ESI†). The molar ratio of elemental Co to Ni in the NCCO is ∼1.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, close to the designed proportion in the precursor solution, according to the XRFS analysis. A typical wide angle XRD (WAXRD) pattern of the as-synthesized NCCO product (Fig. S1, ESI†) demonstrates the formation of a predominant solid-solution phase of NiCO3 (JCPDS file no. 78-0210) and CoCO3 (JCPDS file no. 11-0692) with same rhombohedral structure of R
1, close to the designed proportion in the precursor solution, according to the XRFS analysis. A typical wide angle XRD (WAXRD) pattern of the as-synthesized NCCO product (Fig. S1, ESI†) demonstrates the formation of a predominant solid-solution phase of NiCO3 (JCPDS file no. 78-0210) and CoCO3 (JCPDS file no. 11-0692) with same rhombohedral structure of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (167) and similar lattice constants.22 Careful examination further reveals just one co-existing phase of orthorhombic Co(CO3)0.5(OH)·0.11H2O (JCPDS file no. 11-0692) in the NCCO, with a solubility product constant (∼1.4 × 10−14) that is much smaller than its Ni-based counterpart (∼6.6 × 10−9). The hydrothermal process is subsequently carried out to chemically convert the NCCO into black NCCS (inset in Fig. 1b). Herein inexpensive Na2S serves as a sulfurizing agent for successful phase transformation from the NCCO to thermodynamically favored NCCS via a simple ion-exchange reaction. Fig. 1b displays the WAXRD pattern of the final NCCS to identify its specific crystallographic phase and structure. All the discernable diffraction peaks, as shown in Fig. 1b, suggest that it is composed of cubic NiCo2S4 phase (JCPDS file no. 73-1704, Fd
c (167) and similar lattice constants.22 Careful examination further reveals just one co-existing phase of orthorhombic Co(CO3)0.5(OH)·0.11H2O (JCPDS file no. 11-0692) in the NCCO, with a solubility product constant (∼1.4 × 10−14) that is much smaller than its Ni-based counterpart (∼6.6 × 10−9). The hydrothermal process is subsequently carried out to chemically convert the NCCO into black NCCS (inset in Fig. 1b). Herein inexpensive Na2S serves as a sulfurizing agent for successful phase transformation from the NCCO to thermodynamically favored NCCS via a simple ion-exchange reaction. Fig. 1b displays the WAXRD pattern of the final NCCS to identify its specific crystallographic phase and structure. All the discernable diffraction peaks, as shown in Fig. 1b, suggest that it is composed of cubic NiCo2S4 phase (JCPDS file no. 73-1704, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (227)) with a normal spinel crystal structure (Fig. 1c), in which Ni and Co cations occupy the tetrahedral and/or octahedral sites, and a close-packed Co9S8 phase (JCPDS file no. 86-2273, space group, Fd
m (227)) with a normal spinel crystal structure (Fig. 1c), in which Ni and Co cations occupy the tetrahedral and/or octahedral sites, and a close-packed Co9S8 phase (JCPDS file no. 86-2273, space group, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (255), a = b = c = 9.927 Å), where 8/9 of the Co atoms are paired with the S tetrahedron and 1/9 of Co atoms are coordinated to the octahedra (Fig. 1d).26 The anticipated formation of mixed spinel NiCo2S4 should be favored by good homogeneity of the CoCO3 and NiCO3 phases at an atomic and/or nanoscale level in such a solid solution. The cubic Co9S8 can be easily transformed from the Co(CO3)0.5(OH)·0.11H2O phase by a hydrothermal ion-exchange reaction process, as reported previously.27,28
m (255), a = b = c = 9.927 Å), where 8/9 of the Co atoms are paired with the S tetrahedron and 1/9 of Co atoms are coordinated to the octahedra (Fig. 1d).26 The anticipated formation of mixed spinel NiCo2S4 should be favored by good homogeneity of the CoCO3 and NiCO3 phases at an atomic and/or nanoscale level in such a solid solution. The cubic Co9S8 can be easily transformed from the Co(CO3)0.5(OH)·0.11H2O phase by a hydrothermal ion-exchange reaction process, as reported previously.27,28
The chemical bonding states of each element on the surface of the resulting NCCS sample were evaluated by the core-level XPS technique, and the corresponding XPS information and fitted profiles by using a Gaussian fitting method in the Co, Ni and S regions are shown in Fig. 2a–c. The Co 2p and Ni 2p XPS spectra are fitted well by considering two spin–orbit doublets and two shake-up satellites (identified as “Sat.”). As evident in the Co 2p spectrum (Fig. 2a), the two strong peaks centered at binding energies (BEs) of ∼781.6 and ∼797.5 eV are typically ascribed to the Co(II) species, and the other two at ∼778.8 and ∼793.8 eV are particularly characteristic of elemental Co(III), which are greatly consistent with those for NiCo2S4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29–31 and Co9S8.16,32,33 In the Ni 2p spectrum, two doublets located at BEs of ∼855 and ∼873 eV, as seen in Fig. 2b, are attributed to Ni 2p3/2 and Ni 2p1/2, respectively. The fitted peaks at ∼854.8 and ∼871.8 eV, and the other two at ∼856.1 and ∼873.9 eV correspond to a 2p level splitting of 17.0 and 17.8 eV, respectively, verifying two sorts of co-existing oxidation states of Ni(II) and Ni(III).30,31Fig. 2c demonstrates distinctive contributions of S 2p1/2 (∼161.7 eV) and S 2p3/2 (∼162.8 eV) in the core-level S 2p region.34,35 Another S 2p peak around 168.9 eV is assigned to the surface sulfur species of certain higher oxidation states.34 One should note that the atomic percentages of the Ni(III) and Co(III) species are approximately 38.0 and ∼8.1 at%, respectively, in the NCCS as calculated from the fitted profile areas in Fig. 2a and b. The presence of Ni3+ can commonly result in extra electrons as n-type doping while the existence of Co2+ leads to extra holes as p-type doping. As a consequence, more Ni3+/Co3+ species would result in higher electronic conductivity of the electrode as a result of the self-doping effect.36
29–31 and Co9S8.16,32,33 In the Ni 2p spectrum, two doublets located at BEs of ∼855 and ∼873 eV, as seen in Fig. 2b, are attributed to Ni 2p3/2 and Ni 2p1/2, respectively. The fitted peaks at ∼854.8 and ∼871.8 eV, and the other two at ∼856.1 and ∼873.9 eV correspond to a 2p level splitting of 17.0 and 17.8 eV, respectively, verifying two sorts of co-existing oxidation states of Ni(II) and Ni(III).30,31Fig. 2c demonstrates distinctive contributions of S 2p1/2 (∼161.7 eV) and S 2p3/2 (∼162.8 eV) in the core-level S 2p region.34,35 Another S 2p peak around 168.9 eV is assigned to the surface sulfur species of certain higher oxidation states.34 One should note that the atomic percentages of the Ni(III) and Co(III) species are approximately 38.0 and ∼8.1 at%, respectively, in the NCCS as calculated from the fitted profile areas in Fig. 2a and b. The presence of Ni3+ can commonly result in extra electrons as n-type doping while the existence of Co2+ leads to extra holes as p-type doping. As a consequence, more Ni3+/Co3+ species would result in higher electronic conductivity of the electrode as a result of the self-doping effect.36
 | ||
| Fig. 2 XPS survey spectra and corresponding fitted data of the resultant hetero-NCCS submicro-spindles: (a) Co 2p, (b) Ni 2p and (c) S 2p. | ||
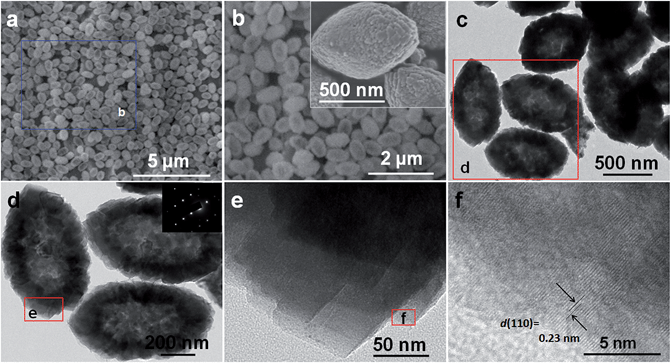
Fig. 3a shows the FESEM image of the as-fabricated NCCO precursor. A large quantity of uniform spindle-shaped products are obtained with high yield through the scalable solvothermal process, which can be further confirmed by a top-view FESEM image with an even larger area of vision (Fig. S2, ESI†). A narrow size distribution for the well-developed submicro-spindles of 900 nm or so in length and ∼500 nm in center width can be observed in Fig. 3a and b. Close observation (inset in Fig. 3b) further shows the tiered surface of the NCCS submicro-spindles, suggesting that they are constructed by some sheet-like nano-building blocks. A representative TEM image (Fig. 3c) reveals the hollow spindle structure for the resulting NCCO sample, as indicated by the strong center–edge contrast. The high-magnification TEM image (Fig. 3d), recorded on the red rectangle region in Fig. 3c, shows a complete void interior with a wall thickness of ∼150 nm. Of great interest, the SAED pattern at the top right corner in Fig. 3d exhibits a quasi-single-crystal diffraction pattern with highly ordered spots, detected from the sampling area in Fig. 3d, indicating a typical mesocrystal feature for the as-obtained NCCO.37 Furthermore, closer inspection (Fig. 3e) apparently shows that hollow submicro-spindles are stacked with thin nanosheet (NS) subunits, which is generally consistent with the FESEM observation (the inset in Fig. 3b). Well-defined lattice fringes with a spacing of ∼0.23 nm are clearly visible in the HRTEM image (Fig. 3f), which is assigned to the typical (110) crystalline plane of the rhombohedral NiCO3 and/or CoCO3 phase.
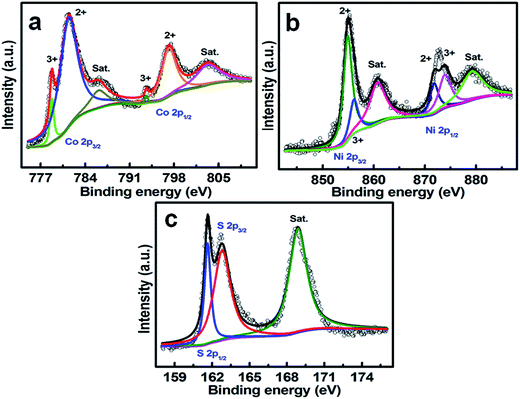
Several further tests were further systematically performed to investigate the influence variables and template-free formation of the hollow spindles. For the case of 20 mmol of NH4HCO3, porous quasi-hollow spheres of sub-micrometer size (∼600 nm) were observed for the NCCO-20 (Fig. S3a and b, ESI†). With the NH4HCO3 amount increasing to 30 mmol, the NCCO-30 sample (Fig. S3c and d, ESI†) shows a solid nanocube-like structure of ∼200 nm in size. Moreover, when the NH4HCO3 decreases to 10 mmol, a solid spindle-like structure is unexpectedly presented (Fig. S4a, ESI†). It is therefore concluded that the amount of NH4HCO3 greatly affects the ultimate morphology of the precursor, and the optimized NH4HCO3 concentration applied here was 15 mmol. In addition, the gradual morphology evolution of the NCCO product with various solvothermal durations was also studied to explore the unique formation process of the hollow structures. Notably, the NCCO-0.5 h sample (Fig. 4a) exhibits an aggregation of nanobelts owing to a spontaneous energy-minimizing self-organization process. With the solvothermal process proceeding for 1.5 h, as a sharp contrast, the product of NCCO-1.5 h presents a mixed structure with solid nanospindles of ∼200 nm in size, and a few nanobelts, as shown in Fig. 4b and c. Careful examination (Fig. 4c) further reveals that these nanospindles are constructed from slim nanobelts of ∼2 nm in diameter and ∼10–50 nm in length. Interestingly, a solid nanospindle morphology assembled by NS subunits is only presented for NCCO-5 h (Fig. 4d) when the solvothermal process duration is extended to 5 h. These findings reveal that the nanobelts should be mutually amalgamated based on the coalescence mechanism24 along with the recrystallization process as well during solvothermal treatment. Thus, solid NSs-constructed nanospindles are observed for the NCCO-5 h. For NCCO-8 h (Fig. 4e), the size of the nanospindles further increases accompanied with the formation of partial hollow cavities. Even larger particles and more cavity space are observed for the NCCO-10 h sample (Fig. 4f) when compared to NCCO-8 h. A similar phenomenon was also found for the resultant NCCO (Fig. 3d) upon prolonging the reaction time up to 20 h. The above discussions indicate that the well-known Ostwald-ripening mechanism along with the inside-out process also occurs over the solvothermal process.38–40 Interestingly, no difference in morphology can be found between the final NCCO (Fig. 3d) and NCCO-25 (Fig. S4b, ESI†), which suggests that the solvothermal duration of 20 h is long enough to produce hollow NCCO specimens.
 | ||
| Fig. 4 Typical TEM images for the intermediates with different hydrothermal durations: (a) NCCO-0.5 h; (b and c) NCCO-1.5 h; (d) NCCO-5 h; (e) NCCO-8 h and (f) NCCO-10 h. | ||
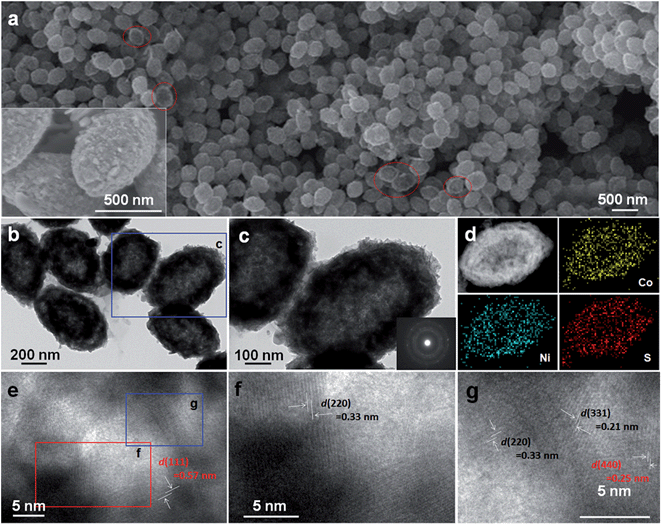
Fig. 5a displays the FESEM image of as-synthesized hetero-NCCS submicro-spindles. The hetero-NCCS specimen has retained the original spindle-like submicro-architecture of the intermediate NCCO without noticeable size alterations or structural collapse after complete sulfidization for 20 h, which is strongly supported by the low-magnification FESEM results (Fig. S5, ESI†). Careful examination (inset in Fig. 5a) further reveals the interior hollow cavities of the resultant hetero-NNCS sample, as discerned from several broken parts marked by red ellipses. TEM microscopy was used to elucidate the microstructure of the resulting hetero-NCCS more clearly. The hollow hetero-NCCS product with a relatively rough surface is shown in Fig. 5b, which is in good agreement with the FESEM picture with high magnification (see the inset in Fig. 5a). The interior-cavity space turns out to be even larger, and the thinner shell of ∼100 nm average thickness appears clearly, as seen from the close-up view in Fig. 5c. The SAED pattern (inset in panel (c)) with a series of concentric rings indicates the polycrystalline characteristics of the resulted hetero-NCCS sample. Typical TEM energy dispersive spectrometer (EDS) elemental (Ni/Co/S) mapping analysis for a single NCCS spindle is shown in Fig. 5d, revealing the co-existence and homogeneous distribution of Ni, Co and S species in the whole hollow NCCS submicro-spindle. The brighter contrast in the magnified TEM image (Fig. 5e) further reveals that the unique hetero-NCCS submicro-spindle consists of interconnected primary nanoparticle (NP) blocks with desirable crystallinity, whereby a large quality of mesopores of ∼3–7 nm in size are created between adjacent nanocrystallites, confirming the good electrolyte permeability of the thin nanoshell. This attractive structural feature would be favorable for the generation of more electroactive sites and convenient mass transport for improved electrochemical energy storage. HRTEM images (Fig. 5e–g) further demonstrate that the well-developed NP subunits with clear lattice fringes are attached to each other in various orientations. As shown in Fig. 5e, lattice fringes can be seen clearly with a spacing of ∼0.57 nm, which is reasonably ascribed to the interplanar distance of the (111) crystalline plane of the Co9S8. Further examination of Fig. 5f, which is to a magnified image of the red square region in Fig. 5e, shows discernible lattice fringes with the spacing of ∼0.33 nm, which can be attributed to the (220) crystalline facet of the spinel NiCo2S4. As seen in Fig. 5g, taken from the blue rectangle region in Fig. 5e, well-defined lattice fringes are observed in three regions with spacings of ∼0.21, ∼0.33, and ∼0.25 nm, which can be indexed to the (331) and (220) planes of the NiCo2S4, and the (440) crystalline facet of the Co9S8 phase, respectively. The above observations confirm that the two nano-phases of NiCo2S4 and Co9S8 are well-dispersed with homogeneous hetero-interfaces at the nanoscale.
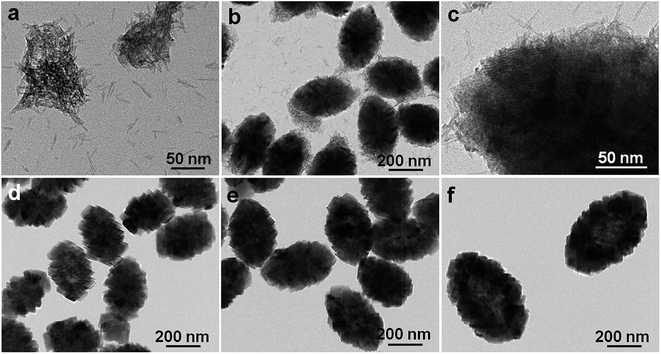
The BET specific surface area (SSA) and specific pore structure of the hollow mesoporous hetero-NCCS were probed with physicochemical N2 sorption isotherm measurements. As plotted in Fig. 6a, the typical isotherms of the hetero-NCCS submicro-spindles can be classified rationally as type IV with a H3-type hysteresis loop in the relative pressure range of 0.4–0.96 P/P0, according to the IUPAC classification, showing its unique mesoporous characteristics.41 This should be mainly contributed by the thin porous shell of the hetero-NCCS sample. Corresponding fitted analysis with the BET equation indicates a BET SSA of ∼86 m2 g−1 and pore volume of ∼0.29 m3 g−1 for the hollow hetero-NCCS product. The average pore size of the hollow hetero-NCCS is calculated as ∼4.8 nm by the BJH method, which is corroborated well by the PSD curve (Fig. 6b).
 | ||
| Fig. 6 (a) N2 adsorption–desorption isotherms and (b) corresponding PSD data of the as-prepared hollow mesoporous hetero-NCCS submicro-spindles. | ||
3.2. Three-electrode electrochemical performance of hollow mesoporous hetero-NCCS submicro-spindles
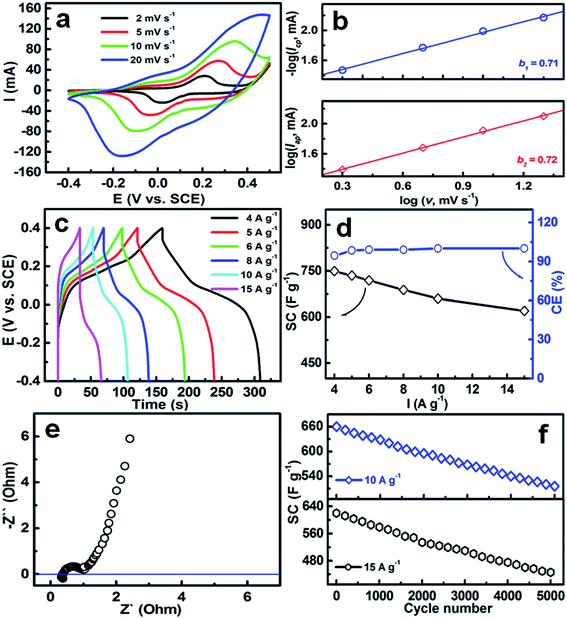
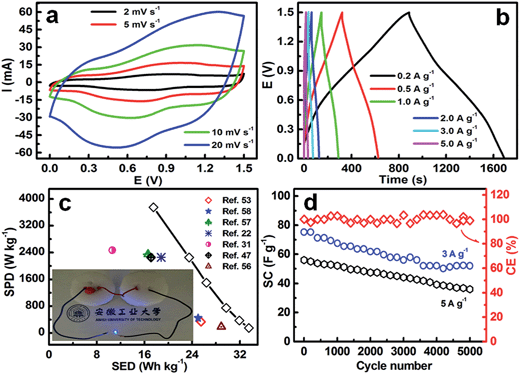
Benefiting from these compelling structural/compositional/componental advantages above, even better electrochemical performance can be expected for the hollow mesoporous hetero-NCCS electrode. A standard three-electrode configuration was first utilized to investigate its electrochemical capacitance via CV, galvanostatic CP, and EIS measurements at RT. Fig. 7a shows typical CV curves at various scanning rates from 2 to 20 mV s−1. As expected, a couple of prominent faradaic redox peaks are apparent within the potential window from −0.4 to 0.5 V (vs. SCE), indicating the good pseudocapacitive characteristics of the hetero-NCCS electrode, which originate from the successive faradaic reactions associated with M–S/M–S–OH (M = Ni and/or Co).42,43 It is because of the contiguous redox potentials of the two that they overlap, and just a pair of faradaic redox peaks are detected herein.43 As established previously, CV is an efficient electrochemical tool to estimate the reaction kinetics of one electrode. The voltammetric response of any electroactive material at various scanning rates can be described in theory as follows:44–46| I = avb | (5) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I = b I = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I vs. log
I vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) according to eqn (6). With regard to a classic capacitive process, b is generally calculated as 1.0. The conventional electrodes of a rechargeable second battery exhibit a b value of ∼0.5 over a wide scanning rate range. As illustrated in Fig. 7b, the redox reactions involved in the hollow mesoporous hetero-NCCS electrode in aqueous KOH electrolyte give b values of ∼0.71 and ∼0.72 for electrochemically anodic and cathodic (i.e., charging and discharging) processes, respectively. Moreover, the b values around the cathodic peaks, such as 0.1 and 0.0 V (vs. SCE), are estimated as ∼0.77 and ∼0.67, respectively, confirming the pseudocapacitive behavior of the hollow hetero-NCCS electrode.44
v) according to eqn (6). With regard to a classic capacitive process, b is generally calculated as 1.0. The conventional electrodes of a rechargeable second battery exhibit a b value of ∼0.5 over a wide scanning rate range. As illustrated in Fig. 7b, the redox reactions involved in the hollow mesoporous hetero-NCCS electrode in aqueous KOH electrolyte give b values of ∼0.71 and ∼0.72 for electrochemically anodic and cathodic (i.e., charging and discharging) processes, respectively. Moreover, the b values around the cathodic peaks, such as 0.1 and 0.0 V (vs. SCE), are estimated as ∼0.77 and ∼0.67, respectively, confirming the pseudocapacitive behavior of the hollow hetero-NCCS electrode.44
The electrochemical performance of the hollow mesoporous hetero-NCCS was further examined by means of galvanostatic discharge/charge technique at RT to obtain SC values. Fig. 7c shows typical CP plots within a wide current density range from 4 to 15 A g−1. The non-linear shape of these galvanostatic curves at various rates verifies the typical pseudocapacitive nature again, resulting from inherent faradaic reactions with multi-valence interconversions from two redox couples of Co(II)/Co(III)/Co(IV) and Ni(II)/Ni(III), which is consistent with the CV analysis above (Fig. 7a). The SCs of the hollow hetero-NCCS electrode are calculated as ∼749, ∼735, ∼719, ∼688, ∼660 and ∼620 F g−1, as displayed in Fig. 7d, corresponding to current densities of 4, 5, 6, 8, 10 and 15 A g−1, respectively. The SC retention of ∼82.7% in such a wide current range reveals outstanding power behavior of the unique hetero-NCCS electrode with a mass loading of 5 mg cm−2, which can be ascribed to its low internal resistance (∼0.35 ohm) and small charge-transfer resistance (∼0.59 ohm) in the faradaic redox process, as estimated from the Nyquist plot (Fig. 7e). Of particular note, the electronic conductivity of the hetero-NCCS is better than that of single-component hollow NiCo2S4 nanoboxes (NBs) with higher atomic content of Co(III) (∼32.3 at%) and Ni(III) (∼65.6 at%) species, and a higher SC degradation of ∼38% is found for the hollow NiCo2S4 NBs with the same mass loading of 5 mg cm−2 when the current rate increases from 4 to 10 A g−1.47 The observation here partially verifies the positive contribution of the NiCo2S4/Co9S8 hetero-interfaces to the enhanced electronic conductivity of the NCCS electrode. The SCs achieved by the hollow hetero-NNCS submicro-spindles are much higher than those for other NiCo2S4 electrodes with lower mass loadings (∼1–4 mg cm−2) and Co9S8 electrodes (see Table S1, ESI†), and even comparable to the state-of-the-art RuO2 electrode.48 Also note that the EIS “small-signal” capacitance of the unique hollow hetero-NCCs is ∼552 F g−1 (f = 0.01 Hz) from the imaginary component of the Nyquist data (Fig. 7e). High electrochemical reversibility of the pseudocapacitive hetero-NCCS electrode can be authenticated by the corresponding CE values of ∼96%, ∼98%, ∼99%, ∼99%, ∼100% and ∼100% at current rates of 4, 5, 6, 8, 10 and 15 A g−1, respectively.
Fig. 7f shows the long-duration cycling performance of the hollow hetero-NCCS electrode at high current rates of 10 and 15 A g−1 up to 5000 consecutive cycles. With uninterruptedly cycling, the SC decreases gradually for both two current rates. Note that the capacitive degradation is ∼22% of the initial SC at a current density of 10 A g−1, corresponding to an average SC decay of around 0.0044% per cycle. A SC retention of about 72% can be found at an even higher rate of 15 A g−1 (i.e., 75 mA cm−2) after cycling for 5000 times. The cycling behavior evaluation demonstrates the desirable high-rate electrochemical stability of our hollow mesoporous hetero-NCCS electrode. With its electrochemical behavior in mind, we strongly envision that the hollow mesoporous hetero-NCCS electrode can be expected to be a powerful electrode candidate for high-performance ESC applications.
3.3. Electrochemical behavior of the AC//hollow mesoporous hetero-NCCS asymmetric supercapacitor
We assembled an asymmetric device by employing as-prepared hetero-NCCS submicro-spindles and AC face-to-face as the positive and negative electrodes, respectively, to explore its practical potential application. Typical CV responses of the AC//hetero-NCCS asymmetric supercapacitor at various sweeping rates of 2, 5, 10 to 20 mV s−1 are shown in Fig. 8a. The electrochemical I–E profiles of the hybrid cell, as shown in Fig. 8a, are nearly symmetric with the respect to the zero-current benchmark, suggesting the exceptional electrochemical behavior of the hybrid supercapacitors. Owing to the lower voltage (−1.0 V, vs. SCE) of the negative AC22 and the upper voltage (0.5 V, vs. SCE) of the hollow hetero-NCCS electrode, an electrochemically stable potential window with an upper limit of 1.5 V is observed for our asymmetric AC//hollow hetero-NCCS device. The upper voltage limit of 1.5 V observed for the unique hybrid device is somewhat larger than other asymmetric systems, such as AC//Co3O4-rGO (1.45 V),49 AC//CoAl double hydroxide (1.2 V),50 and AC//RuO2–TiO2 nanotubes (NTs) (1.4 V),51 which is of great significance in upgrading the SED of a full device, because the SED of any cell is proportional to the square of the upper cut-off voltage.49–52The galvanostatic charge/discharge plots of the hybrid capacitor were measured with a wide current rate range from 0.2 to 5 A g−1 in the electrochemical potential window of 0.0–1.5 V, and the corresponding CP plots are shown in Fig. 8b. A nearly linear variation of the electrochemical potential is found for the hybrid AC//hetero-NCCS supercapacitor over the charge/discharge processes, exhibiting its outstanding supercapacitive performance. The hybrid device delivers large SCs of ∼107, ∼102, ∼95, ∼85, ∼75 and ∼56 F g−1 (Fig. S6, ESI†), at the active mass-normalized current densities of 0.2, 0.5, 1.0, 2.0, 3.0 and 5.0 A g−1, respectively, which are even higher than those for other NiCo2S4-based hybrid capacitors, for instance, AC//NiCo2S4 NBs (∼88 F g−1 at ∼0.2 A g−1),47 AC//NiCo2S4 NSs (∼72 F g−1 at 1.25 A g−1),53 AC//porous NiCo2S4 NSs (∼80 F g−1 at ∼3.6 A g−1),54 AC//NiCo2S4 NTs (∼67 F g−1 at 0.5 A g−1),55 AC//core–shell NiCo2S4@Co(OH)2 NTs (∼101 F g−1 at 0.5 A g−1),55 and AC//NiCo2S4 ellipsoids (∼92 F g−1 at 0.5 A g−1).56 When the current is increased from 0.2 to 5.0 A g−1, the SC still remains at ∼52.3% of that at a current density of 0.2 A g−1.
The SED and SPD of the asymmetric cell were further calculated based on the CP data in Fig. 8b, and a typical Ragone plot at various current rates is displayed in Fig. 8c. The asymmetric supercapacitor exhibits a maximum energy density of ∼33.5 W h kg−1 at a current rate of 0.2 A g−1, and the corresponding power density is ∼150 W kg−1. After a 25-times increase in the current density, the power density can reach ∼3.75 kW kg−1, while the SED can still remain as large as ∼17.5 W h kg−1, showing its great superiority for ESCs. Our AC//hollow hetero-NCCS hybrid cell exhibits more appealing electrochemical performance when compared to other NiCo2S4-based hybrid devices (see the inset data in Fig. 8c), such as AC//NiCo2S4 NBs (∼17.1 W h kg−1 at ∼2.25 kW kg−1),47 AC//NiCo2S4 ellipsoids (∼28.9 W h kg−1 at ∼187.5 W kg−1),56 graphene//NiCo2S4 NTs arrays on Ni foam (∼16.6 W h kg−1 at ∼2.35 kW kg−1),57 AC//NiCo2S4 (∼25 W h kg−1 at ∼447 W kg−1),58 AC//NiCo2S4 NSs (∼25.5 W h kg−1 at ∼334 W kg−1),51 AC//Ni–Co–S (∼18.8 W h kg−1 at ∼2.26 kW kg−1)22 and C//NiCo2S4 (∼10.6 W h kg−1 at ∼2.47 kW kg−1),31 and other asymmetric ESCs (see Table S2, ESI†). In addition, a 3.0 V blue LED (inset in Fig. 8c) can be actuated by our two hybrid capacitors in series here.
Long-term cycling stability is another key parameter to assess high-performance ESCs. The cycling performance of the AC//hetero-NCCS asymmetric supercapacitor was examined at a large current density of 3 A g−1 over 5000 continuous cycles. As shown in Fig. 8d, the SC of the hybrid device initially drops gradually, and then remains relatively stable during the subsequent charging/discharging cycles. A good SC retention of ∼70% of the initial value can be achieved after cycling up to 5000 times, that is, the average SC degradation is just ∼0.006% per cycle, which is much better than those for other reports including AC//NiCo2S4 NBs (∼75% at 2 A g−1 after 5000 cycles),47 AC//NiCo2S4 NTs (∼65.5% at 1 A g−1 after 5000 cycles),55 AC//core–shell NiCo2S4@Co(OH)2 NTs (∼70.1% at 1 A g−1 after 5000 cycles),55 AC//NiCo2S4 nanowires (∼73.1% at 32 mA cm−2 after 3000 cycles),59 and AC//carbon–NiCo2S4 NSs (∼71.9% at 150 mA cm−2 after 2500 cycles).60 Our hybrid cell still can display a SC retention of ∼65% at a high rate of 5 A g−1 (i.e., 105 mA cm−2) after 5000 cycles, suggesting good electrochemical stability of our hybrid. Furthermore, the asymmetric cell maintains a high CE of ∼100% over continuous cycling at 3 A g−1, as shown in Fig. 8d, demonstrating good electrochemical reversibility of the as-fabricated hybrid device. From the detailed electrochemical analysis above, we can safely conclude that the hollow mesoporous hetero-NCCS submicro-spindles hold huge practical potential in advanced next-generation ESCs.
4. Conclusions
We herein reported a facile but efficient template-free synthetic methodology to fabricate hollow mesoporous hetero-NCCS submicro-spindles in high yield with well-dispersed NiCo2S4 and Co9S8 nano-subunits at the nanoscale, and further utilized it as an electrode material for advanced ESCs. Systematic investigations shed light on the intrinsic template-free formation mechanism of the hollow mesoporous submicro-spindles. Benefiting from multiple synergetic effects of inherent structural/compositional/componental advantages, the resultant hollow mesoporous hetero-NCCS submicro-spindles delivered an encouraging pseudocapacitance of ∼749 F g−1 at 4 A g−1 and even ∼620 F g−1 at a higher rate of 15 A g−1, and good electrochemical stability at high rates. An AC//hollow hetero-NCCS asymmetric cell was assembled, and exhibited a large energy density of ∼33.5 W h kg−1 at a power density of ∼150 W kg−1, and desirable long-term cycling performance with a SC retention of ∼65% at a high current rate of 5 A g−1 after a 5000-cycle test. These electrochemical results further confirmed that the hollow mesoporous hetero-NCCS submicro-spindles would be a promising electrode platform for next-generation ESCs. The smart design, and in-depth understanding of the fundamental relationship between intrinsic structure/composition/component and electrochemical capacitances is highly significant for general and fine regulation of electrode materials for advanced ESCs, and even for Li-ion batteries.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (No. 51572005, 51502003), Anhui Province Funds for Distinguished Young Scientists (No. 1508085J09), the Natural Science Foundation of Anhui Province (No. 1508085ME106), the Foundation for Young Talents in College of Anhui Province and Graduation Innovation Research Foundation of Anhui University of Technology (2015076, 2015088).References
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 653 CrossRef PubMed.
- S. E. Chun, B. Evanko, X. F. Wang, D. Vonlanthen, X. L. Ji, G. D. Stucky and S. W. Boettcher, Nat. Commun., 2015, 6, 7818 CrossRef CAS PubMed.
- B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer Academic/Plenum Publishers, New York, 1999 Search PubMed.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- C. Z. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879 RSC.
- S. Peng, L. Li, C. Li, H. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Yan, Chem. Commun., 2013, 49, 10178 RSC.
- L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 3711 CrossRef CAS PubMed.
- L. F. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. G. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- C. Z. Yuan, J. Y. Li, L. R. Hou, X. G. Zhang, L. F. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS.
- J. Xiao, L. Wan, S. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831 CrossRef CAS PubMed.
- R. J. Zou, Z. Y. Zhang, M. F. Yuen, J. Q. Hu, C. S. Lee and W. J. Zhang, Sci. Rep., 2015, 5, 7862 CrossRef PubMed.
- R. Ramachandran, M. Saranya, C. Santhosh, V. Velmurugan, B. P. C. Raghupathy, S. K. Jeong and A. N. Grace, RSC Adv., 2014, 4, 21151 RSC.
- H. Li, Y. H. Gao, Y. D. Shao, Y. T. Su and X. W. Wang, Nano Lett., 2015, 15, 6689 CrossRef CAS PubMed.
- Z. M. Zhang, Q. Wang, C. J. Zhao, S. D. Min and X. Z. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 4861 CAS.
- L. Yin, L. Q. Wang, X. H. Liu, Y. S. Gai, L. H. Su, B. H. Qu and L. Y. Gong, Eur. J. Inorg. Chem., 2015, 14, 2457 CrossRef.
- X. Hong, J. Kim, S. Shi, Y. Zhang, C. Jin, Y. Sun, S. Tongay, J. Wu, Y. Zhang and F. Wang, Nat. Nanotechnol., 2008, 9, 682 CrossRef PubMed.
- J. Nishitani, K. Yu and W. Walukiewicz, Appl. Phys. Lett., 2014, 105, 132103 CrossRef.
- Y. Zheng, T. F. Zhou, C. F. Zhang, J. F. Mao, H. K. Liu and Z. P. Guo, Angew. Chem., Int. Ed., 2016, 55, 3408 CrossRef CAS PubMed.
- L. R. Hou, L. Lian, L. H. Zhang, G. Pang, C. Z. Yuan and X. G. Zhang, Adv. Funct. Mater., 2015, 25, 238 CrossRef CAS.
- J. Wang, Y. Zhou and Z. Shao, Electrochim. Acta, 2013, 97, 386 CrossRef CAS.
- H. Hua, S. J. Liu, Z. Y. Chen, R. Q. Bao, Y. Y. Shi, L. R. Hou, G. Pang, K. N. Hui, X. G. Zhang and C. Z. Yuan, Sci. Rep., 2016, 6, 20973 CrossRef CAS PubMed.
- Z. Y. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903 CrossRef CAS PubMed.
- C. Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772 RSC.
- N. N. Wang, X. J. Ma, H. Y. Xu, L. Chen, J. Yue, F. E. Niu, J. Yang and Y. T. Qian, Nano Energy, 2014, 6, 193 CrossRef CAS.
- X. H. Rui, H. T. Tan and Q. Y. Yan, Nanoscale, 2014, 6, 9889 RSC.
- J. W. Yu, H. Z. Wan, J. J. Jiang, Y. J. Ruan, L. Miao, L. Zhang, D. D. Xia and K. Xu, J. Electrochem. Soc., 2014, 161, A966 Search PubMed.
- X. H. Xia, C. R. Zhu, J. S. Luo, Z. Y. Zeng, C. Guan, C. H. Ng, H. Zhang and H. J. Fan, Small, 2014, 4, 766 CrossRef PubMed.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981 CAS.
- C. Z. Yuan, J. Y. Li, L. R. Hou, J. D. Lin, X. G. Zhang and S. L. Xiong, J. Mater. Chem. A, 2013, 1, 11145 CAS.
- W. Kong, C. C. Lu, W. Zhang, J. Pu and Z. H. Wang, J. Mater. Chem. A, 2015, 3, 12452 CAS.
- B. Y. Hu, Z. Z. Jing, J. J. Fan, G. D. Yao and F. M. Jin, Catal. Today, 2016, 263, 128 CrossRef CAS.
- L. L. Feng, M. H. Fan, Y. Y. Wu, Y. P. Liu, G. D. Li, H. Chen, W. Chen, D. J. Wang and X. X. Zou, J. Mater. Chem. A, 2016, 4, 6860 CAS.
- W. T. Wei, L. W. Mi, Y. Cao, Z. Zheng, W. H. Chen and X. X. Guan, Chem. Mater., 2014, 26, 3418 CrossRef CAS.
- V. H. Nguyen and J. J. Shim, Electrochim. Acta, 2015, 166, 302 CrossRef CAS.
- X. Wang, C. Yan, A. Sumboja, J. Yan and P. S. Lee, Adv. Energy Mater., 2014, 4, 1301240 CrossRef.
- L. R. Hou, C. Z. Yuan, L. Yang, L. F. Shen, F. Zhang and X. G. Zhang, CrystEngComm, 2011, 13, 6130 RSC.
- X. W. Lou, L. A. Archer and Z. C. Yang, Adv. Mater., 2008, 20, 3987 CrossRef CAS.
- B. Wang, H. B. Wu, L. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 4165 CrossRef CAS PubMed.
- C. Z. Yuan, J. Y. Li, L. R. Hou, L. H. Zhang and X. G. Zhang, Part. Part. Syst. Charact., 2014, 31, 657 CrossRef CAS.
- Y. J. Li, G. L. Wang, T. Wei, Z. J. Fan and P. Yan, Nano Energy, 2015, 19, 165 CrossRef.
- Y. L. Xiao, Y. Lei, B. Z. Zheng, L. Gu, Y. Y. Wang and D. Xiao, RSC Adv., 2015, 5, 21604 RSC.
- Y. Lei, J. Li, Y. Y. Wang, L. Gu, Y. F. Chang, H. Y. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 1773 CAS.
- S. Y. Dong, L. F. Shen, H. S. Li, P. Nie, Y. Y. Zhu, Q. Sheng and X. G. Zhang, J. Mater. Chem. A, 2015, 3, 21277 CAS.
- X. D. Dong, L. Chen, J. Y. Liu, S. Haller, Y. G. Wang and Y. Y. Xia, Sci. Adv., 2016, 2, e1501038 Search PubMed.
- I. E. Rauda, V. Augustyn, B. Dunn and S. H. Tolbert, Acc. Chem. Res., 2013, 46, 1113 CrossRef CAS PubMed.
- L. R. Hou, H. Hua, R. Q. Bao, Z. Y. Chen, C. Yang, S. Q. Zhu, G. Pang, L. N. Tong, C. Z. Yuan and X. G. Zhang, ChemPlusChem, 2016, 81, 557 CrossRef CAS.
- W. Wang, S. R. Guo, L. Lee, J. B. Zhong, Z. Favors, F. Zaera, M. Ozkan and C. S. Ozkan, Sci. Rep., 2014, 4, 4452 Search PubMed.
- C. Z. Yuan, L. H. Zhang, L. R. Hou, G. Pang and W. C. Oh, RSC Adv., 2014, 4, 14408 RSC.
- Y. G. Wang, L. Chen and Y. Y. Xia, J. Power Sources, 2006, 153, 191 CrossRef CAS.
- Y. G. Wang, Z. D. Wang and Y. Y. Xia, Electrochim. Acta, 2005, 50, 5641 CrossRef CAS.
- C. Z. Yuan, B. Gao and X. G. Zhang, J. Power Sources, 2007, 173, 606 CrossRef CAS.
- Z. B. Wu, X. L. Pu, X. B. Ji, Y. R. Zhu, M. J. Jing, Q. Y. Chen and F. P. Jiao, Electrochim. Acta, 2015, 174, 238 CrossRef CAS.
- X. M. Li, Q. G. Li, Y. Wu, M. C. Rui and H. B. Zeng, ACS Appl. Mater. Interfaces, 2015, 7, 19316 CAS.
- R. Li, S. L. Wang, Z. C. Huang, F. X. Lu and T. B. He, J. Power Sources, 2016, 312, 156 CrossRef CAS.
- L. R. Hou, R. Q. Bao, Z. Y. Chen, M. Rehan, L. N. Tong, G. Pang and C. Z. Yuan, Electrochim. Acta, 2016, 214, 76 CrossRef CAS.
- H. C. Chen, J. J. Jiang, L. Zhang, D. D. Xia, Y. D. Zhao, D. Q. Guo, T. Qi and H. Z. Wan, J. Power Sources, 2014, 254, 249 CrossRef CAS.
- H. L. Wang, C. M. B. Holt, Z. Li, X. H. Tan, B. S. Amirkhiz, Z. W. Xu, B. C. Olsen, T. Stephenson and D. Mitlin, J. Nano Res., 2012, 5, 605 CrossRef CAS.
- Y. H. Li, L. J. Cao, L. Qiao, M. Zhu, Y. Yang, P. Xiao and Y. H. Zhang, J. Mater. Chem. A, 2014, 2, 6540 CAS.
- H. Wang, C. Wang, C. Qing, D. M. Sun, B. X. Wang, G. Qu, M. Sun and Y. W. Tang, Electrochim. Acta, 2015, 174, 1104 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD, FESEM and TEM images, SAED pattern, electrochemical data of the controlled experiments, and corresponding electrochemical comparisons. See DOI: 10.1039/c6ta05788h |
| This journal is © The Royal Society of Chemistry 2017 |