Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ligand removal from CdS quantum dots for enhanced photocatalytic H2 generation in pH neutral water†
Christina M.
Chang‡
,
Katherine L.
Orchard‡
,
Benjamin C. M.
Martindale
and
Erwin
Reisner
*
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK. E-mail: reisner@ch.cam.ac.uk; Web: http://www-reisner.ch.cam.ac.uk
First published on 19th June 2015
Abstract
Ligand-free CdS quantum dots were produced by a reactive ligand stripping procedure and employed for photocatalytic H2 evolution in pH neutral solution. The rate of H2 generation of the ‘bare’ quantum dots was 175 times higher than that of the equivalent mercaptopropionic acid-capped quantum dots in the presence of a cobalt co-catalyst and Na2SO3 as a sacrificial electron donor. Under optimised conditions, a turnover number of 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mol H2 per mol Co and 29
000 mol H2 per mol Co and 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mol H2 per mol CdS quantum dots was achieved after 88 h of UV-free solar light irradiation (λ > 420 nm, 1 Sun intensity). Ligand removal is therefore a potent method to substantially enhance the photocatalytic performance of quantum dot systems.
000 mol H2 per mol CdS quantum dots was achieved after 88 h of UV-free solar light irradiation (λ > 420 nm, 1 Sun intensity). Ligand removal is therefore a potent method to substantially enhance the photocatalytic performance of quantum dot systems.
Introduction
Converting the energy in sunlight into chemical fuels is a potential solution to meet our need for sustainable energy storage.1–3 Many synthetic systems have been developed with the hope of emulating natural photosynthesis to produce renewable fuels such as H2. Such artificial photosynthetic systems typically consist of a photosensitiser – such as a molecular dye or a semiconductor (nano)particle – coupled to an inorganic, organometallic or enzymatic catalyst, which carries out the fuel-producing redox reaction.4–7Semiconductor quantum dots (QDs) are well suited as light absorbers for photocatalysis due to their high light absorption coefficient, high surface area, tuneable optical and electronic properties, and relatively high photostability compared to molecular dyes.7–9 Although photocatalytic H2 generation systems using bulk and nanostructured metal chalcogenides have been studied for many years,10–15 systems using QDs have been pioneered only relatively recently, employing CdX (X = S, Se, Te) QDs coupled to a wide range of co-catalysts, including noble metals,16,17 enzymes,18–21 and molecular complexes or salts of iron,22–24 cobalt,25–27 and nickel.28,29
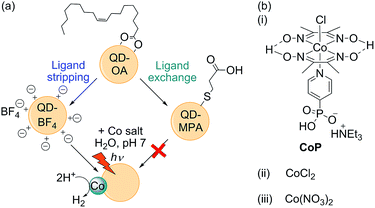
The most active QD-based photocatalytic systems to date operate under either acidic (pH < 5),22–29 or highly alkaline conditions (pH ≥ 11),30 with greatly reduced activity around neutral pH. However, activity close to pH 7 is important for developing sustainable technologies in which, ultimately, river water or seawater could be employed. In addition, enzymes and some synthetic electrocatalysts such as the cobaloxime CoP (Fig. 1) and semiconductor light absorbers such as BiVO4 (for use in tandem with a QD for full water splitting) display optimal activity around pH 7.18,31,32 Therefore, extending the utility of QDs to neutral conditions is an important goal.
QDs used in photocatalytic systems have previously been functionalised with hydrophilic, thiol-based ligands, such as thioglycolic acid (TGA), 3-mercaptopropionic acid (MPA), or dihydrolipoic acid (DHLA), in order to prevent aggregation and to induce solubility in aqueous solution. However, these capping ligands are not innocent and have been proposed to have secondary roles such as acting as a physical33 or electronic barrier.34 They have also been shown to interfere with co-catalysts by denaturing enzymes,19 and by altering metal-based molecular catalysts to form complexes that are either more active (for Ni2+)28 or inactive (for Co2+).27 Moreover, the affinity of thiol ligands for CdX surfaces decreases with decreasing pH,35,36 such that the ligands desorb more readily under commonly-used acidic photocatalytic conditions.
Whereas previous work has sought to improve QD photoactivity at low pH by increasing the binding affinity of the capping ligands,27 here we take the opposite approach and demonstrate that QD activity at neutral pH is dramatically improved by complete removal of the surface ligands. We propose that desorption of surface capping groups is integral to QD/catalyst activity and show that the use of bare QDs results in a two orders of magnitude increase in catalytic rate compared to the equivalent MPA-capped QD/catalyst system.
Experimental section
General materials and methods
Unless otherwise stated, reagents were purchased from commercial suppliers and used as received with the following purities: CdO (99.998%), sulfur (99.998%), oleic acid (OA, 90%), octadecene (ODE, 90%), 3-mercaptopropionic acid (MPA, ≥99%), tetramethylammonium hydroxide, pentahydrate (TMAOH, 99%), trimethyloxonium tetrafluoroborate (96%), sodium sulfite (≥98%), cobalt(II) chloride hexahydrate (98%). Anhydrous solvents were purchased from Acros Organics with the following purities: CHCl3 (99.9%), dimethylformamide (DMF, 99.8%), acetonitrile (ACN, 99.9%). All other organic solvents used were HPLC grade. All aqueous solutions were prepared with distilled water. (Et3NH)[CoIIICl(dmgH)2(pyridyl-4-hydrophosphonate)] (CoP) was prepared according to a literature procedure.37 Unless otherwise stated, all experiments were carried out in air. Na2SO3 buffer solutions were titrated with dilute HCl to the desired pH value at 25 °C.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hexane
1 hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol using excess acetone, centrifuged at 7000 rpm for 3 min, and re-dispersed in hexane. Two further washing steps were carried out using hexane and acetone as solvent and non-solvent, respectively, before finally dispersing in hexane (20 mL).
methanol using excess acetone, centrifuged at 7000 rpm for 3 min, and re-dispersed in hexane. Two further washing steps were carried out using hexane and acetone as solvent and non-solvent, respectively, before finally dispersing in hexane (20 mL).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform
1 chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (10 mL) and the pH was adjusted to 11 with TMAOH. QD-OA solution (2 mL) was added to this mixture and stirred in the dark for two days. The QDs were precipitated with excess acetone and centrifuged (7000 rpm, 3 min). The isolated particles were washed with acetone before being dispersed in water (1 mL).
methanol (10 mL) and the pH was adjusted to 11 with TMAOH. QD-OA solution (2 mL) was added to this mixture and stirred in the dark for two days. The QDs were precipitated with excess acetone and centrifuged (7000 rpm, 3 min). The isolated particles were washed with acetone before being dispersed in water (1 mL).
Photocatalysis experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min). The supernatant was separated and the precipitate washed by re-suspending in water (2 mL) followed by centrifugation (10 min). The precipitate was then suspended in fresh Na2SO3 (0.1 M, pH 7), purged with 2% CH4 in N2 and irradiated for a further 4 h. To avoid any deleterious effects of exposure to air, the isolation and re-suspension procedure was carried out in a nitrogen-filled glovebox.
000 rpm, 10 min). The supernatant was separated and the precipitate washed by re-suspending in water (2 mL) followed by centrifugation (10 min). The precipitate was then suspended in fresh Na2SO3 (0.1 M, pH 7), purged with 2% CH4 in N2 and irradiated for a further 4 h. To avoid any deleterious effects of exposure to air, the isolation and re-suspension procedure was carried out in a nitrogen-filled glovebox.
Results and discussion
Synthesis and surface modification of CdS QDs
As outlined in Fig. 1, oleic acid-capped CdS QDs (QD-OA) were synthesised by a hot injection method,16 and used to prepare both MPA-capped particles (QD-MPA) and ligand-free, charge-stabilised QDs (QD-BF4). QD-MPA were prepared following a standard ligand exchange procedure by treating QD-OA with MPA in basic solution.35 QD-BF4 were prepared following a modified reactive ligand stripping procedure, using [Me3O]BF4 in the presence of dimethylformamide (DMF) to remove the oleic acid groups.38 The ligand stripping process yields well-dispersed individual particles when suspended in polar solvents such as DMF and dimethyl sulfoxide (Fig. S1† for transmission electron microscopy, TEM, image).Fourier transform infrared spectroscopy (FT-IR) of dried QD-BF4 revealed resonances from DMF and (BF4)−, which are the expected surface groups following ligand stripping (Fig. S2†). Ligand stripping did not affect the CdS crystal phase (Fig. S3† for powder X-ray diffraction, XRD), but the absorption maximum changed from λmax = 443 nm to 427 nm (Fig. S4† for UV-vis spectra). This blue-shift corresponds to a decrease in the CdS QD particle size from 5.0 nm to 4.2 nm,39 which is in agreement with TEM measurements (4.5 ± 0.5 nm for QD-OA and 4.1 ± 0.6 nm for QD-BF4). The (BF4)− anion is known to etch CdTe QDs,40 and therefore a similar etching process is a likely reason for the reduced size of QD-BF4.
Photocatalysis experiments
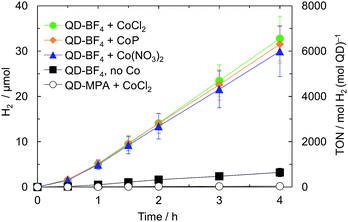
Photocatalysis solutions were prepared by sequentially adding stock solutions of QDs and either CoP, CoCl2 or Co(NO3)2 to a stirred, aqueous solution of the sacrificial electron donor (0.1 M Na2SO3, pH 7). Whereas QD-MPA formed a stable suspension that did not precipitate over the course of the photocatalysis experiment, QD-BF4 rapidly formed a visibly hazy suspension that precipitated if not stirred. FT-IR of QD-BF4 precipitated from aqueous solution shows significantly reduced (BF4)− resonances (Fig. S2†), indicating that most of the counter ions are dissolved in water. Under visible light irradiation (air mass 1.5 G filter, 100 mW cm−2, λ > 420 nm), QD-MPA produced negligible amounts of H2 in the presence and absence of cobalt co-catalysts (Fig. 2, Tables 1 and S1†). In contrast, QD-BF4 was over 60 times more active than QD-MPA, and its activity was further enhanced tenfold by the addition of cobalt species.| Systema | H2/μmol | TOFQDb ± σ/h−1 | TOFCoc ± σ/h−1 |
|---|---|---|---|
| a Values reported for H2 produced after 4 h irradiation at 25 °C (λ > 420 nm, 100 mW cm−2, [QD] = 2 μM, [Co] = 4 μM, 0.1 M Na2SO3, pH 7, 2.5 mL). b Turnover frequency per QD, TOFQD, in mol H2 per mol QD per h. c Turnover frequency per Co, TOFCo, in mol H2 per mol Co per h. | |||
| QD-MPA | 0.05 ± 0.02 | 2.63 ± 0.88 | — |
| QD-MPA/CoCl2 | 0.19 ± 0.03 | 9.36 ± 1.42 | 4.68 ± 0.71 |
| QD-BF4 | 3.22 ± 0.95 | 161 ± 48 | — |
| QD-BF4/CoCl2 | 32.8 ± 4.9 | 1639 ± 246 | 819 ± 123 |
To control for the reduced particle size of QD-BF4 compared to QD-MPA, we capped QD-BF4 with MPA (‘re-capped’ QDs) and compared the activity. The H2 evolution yield of the ‘re-capped’ dots with CoCl2 remained significantly lower than that of the bare QD-BF4 (1.53 ± 0.42 and 32.8 ± 4.9 μmol after 4 h irradiation, respectively; Table S1†), confirming that the primary activity enhancing effect of ligand stripping stems from the lack of MPA surface groups in QD-BF4 and not the decrease in QD size. Furthermore, the presence of small amounts of DMF in the QD-BF4 solutions is not a contributing factor to the difference in activity between QD-BF4 and QD-MPA, since adding an equivalent quantity of DMF to QD-MPA solutions did not influence the activity. Equivalent experiments with commercial CdS (Sigma-Aldrich, 0.6 mg) yielded activity on the same order of magnitude as that of QD-MPA (Table S1,† entry 10). From these results we infer that both the small particle size and the bare CdS surface are necessary for high activity.
The observed photocatalytic activity was independent of the Co species used as co-catalyst (Fig. 1, 2 and Table S1†), which suggests that the same active species is formed in each case. Although CoP has been shown to act as a molecular catalyst on dye-sensitised TiO2,41,42 cobaloximes are known to partially dissociate from a surface or light absorber,42–45 and to fully decompose under highly reducing conditions.46,47 The presence of an induction period followed by linear photostability over hours is in contrast to the behaviour of CoP on dye-sensitised TiO2, and is consistent with decomposition to form a catalytically active deposit.48 We therefore propose that CoP is photodecomposed during an initial activation period, which may result from the high driving force provided by the CdS conduction band (values reported range from −1.7 V to −0.7 V vs. NHE, depending on surface properties and QD diameter).49
The photocatalytic QD-BF4 system was optimised using CoCl2 as the co-catalyst. A [QD]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Co] ratio of 1
[Co] ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, with [QD] = 2 μM, and [Na2SO3] of 1.0 M was found to give optimal H2 evolution activity (Fig. S5–S7 and Table S2† entry 12). A five-fold higher loading of Co did not increase the activity with respect to the QDs. The external quantum efficiency (EQE) of the QD-BF4/CoCl2 system at λ = 420 nm was 7.7 ± 1.4% and remained approximately constant over 3 h (see ESI for details†).
2, with [QD] = 2 μM, and [Na2SO3] of 1.0 M was found to give optimal H2 evolution activity (Fig. S5–S7 and Table S2† entry 12). A five-fold higher loading of Co did not increase the activity with respect to the QDs. The external quantum efficiency (EQE) of the QD-BF4/CoCl2 system at λ = 420 nm was 7.7 ± 1.4% and remained approximately constant over 3 h (see ESI for details†).
The photocatalytic activity is highly sensitive to the solution pH with a clear maximum in activity at pH ∼ 7 (Fig. S8†). The optimum pH correlates with the pKa of the sacrificial electron donor (7.2 for HSO3−),50 but may also be related to other factors such as the degree of protonation of the particle surfaces and the corresponding changes in the QD conduction and valence band energies or a pH-dependent quantum yield of generated photo-reduced species.30,51,52 This pH sensitivity of the QD-BF4/CoCl2 hybrid system was found to be the main factor limiting the long-term stability of the system (Fig. 3). Under continuous solar light illumination (λ > 420 nm), the H2 evolution ceased after approximately 44 h, where a pH of 11.3 ± 0.1 was measured. Adjusting the solution pH again to pH 7 with aqueous HCl restored much of the initial rate of H2 evolution. The activity was not fully restored to the initial rate, which may be due to degradation of the QDs by repeated exposure to air, alkaline solution and/or HCl (for pH adjustment). In contrast, adding Na2SO3 did not cause an increase in activity. After 88 h irradiation, 581 ± 58 μmol H2 was produced, corresponding to a TONCo of 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 5800 and TONQD of 29
000 ± 5800 and TONQD of 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 2900. QD-BF4/CoCl2 is the most active QD/catalyst system at pH 7 to date, with activity on par with – or higher than – the related photocatalytic microstructured nanoparticle/co-catalyst aggregates, formed from MPA-capped CdTe QDs and CoCl2 at pH 4.65 (Table S3†).25
000 ± 2900. QD-BF4/CoCl2 is the most active QD/catalyst system at pH 7 to date, with activity on par with – or higher than – the related photocatalytic microstructured nanoparticle/co-catalyst aggregates, formed from MPA-capped CdTe QDs and CoCl2 at pH 4.65 (Table S3†).25
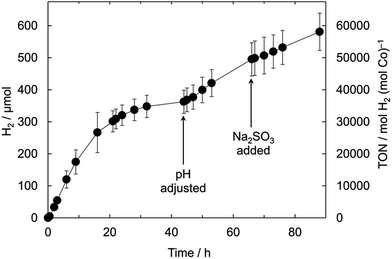 | ||
| Fig. 3 Long-term photoactivity of the optimised QD-BF4/CoCl2 system at 25 °C ([QD-BF4] = 2 μM, [Co] = 4 μM, 1.0 M Na2SO3, pH 7, λ > 420 nm, 100 mW cm−2). | ||
Characterisation of the active catalyst
Inductively-coupled plasma-optical emission spectroscopy (ICP-OES) of both the particles and supernatant isolated from a QD-BF4/CoCl2 solution after 4 h irradiation showed that 87% of the detected Co is attached to the QDs, with a calculated Co loading of ∼0.1 wt% (Table S4†). It is notable that the measured Co content on QD-BF4 is similar to that of the related CdTe/CoCl2 system (0.15 wt%),25 but that the required amount of CoCl2 in solution for our system is much lower, presumably due to the ligand-free CdS surface (2 equivalents per QD in this work compared to 1000 equivalents for CdTe/CoCl2). The TONCo based on total added Co is therefore much higher for our system (TONCo 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 after 21 h cf. 86 for CdTe/CoCl2; Table S3†).
000 after 21 h cf. 86 for CdTe/CoCl2; Table S3†).
Particles isolated from a QD-BF4/CoP solution were found to have a similar Co loading to that of QD-BF4/CoCl2 (Table S4†), supporting the hypothesis that the nature of the active catalyst is independent of the Co species. Additionally, Co was not detected in particles isolated from either QD-MPA/CoCl2 or ‘re-capped’ QD-BF4/CoCl2 solutions, indicating that integration of Co into the particles is necessary for high photocatalytic activity.
Particles isolated from the photocatalysis solution and re-suspended in fresh Na2SO3 retained 90% of their activity (no additional Co, Fig. S9†), confirming that the CdS/Co particles are the active species. An induction period was observed within the first 30 min of irradiation (Fig. 2), indicating that the formation of the active species is a photoactivated process. Stirring the photocatalysis mixture in the dark for 4 h prior to irradiation did not result in a shorter induction period.
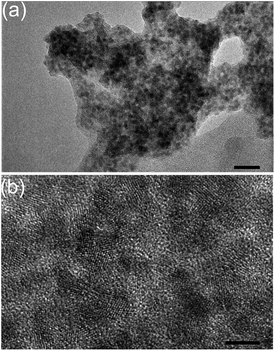
TEM of the particles after photocatalysis revealed aggregates of ∼100 nm that retained a nanocrystalline morphology (Fig. 4), but no additional features due to Co species (e.g. Co0 nanoparticles) were detected. Neither changes in the crystal phase of the CdS nor formation of additional phases were observable by XRD (Fig. S3†). We were unable to determine the oxidation state of the active Co species since the loading of Co was below the detection limit of X-ray photoelectron spectroscopy (XPS).
The oxidation state of bulk and surface CdS may also play an important role in catalysis. A comparison of XPS data for QD-OA, QD-BF4 and QD-BF4/CoCl2 indicated changes in the QD surface chemistry during both ligand stripping and photocatalysis. Firstly, there is a change in the stoichiometry of the particles during ligand-stripping, with QD-OA found to be Cd-rich (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 0.63
S = 0.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.37), whereas QD-BF4 was almost stoichiometric (Cd
0.37), whereas QD-BF4 was almost stoichiometric (Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 0.53
S = 0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.47) and remained so during photocatalysis (Table 2). The Cd(3d) peaks of QD-OA were broadened compared with those of QD-BF4 (Fig. S10 and S11†), and could be fit to two spin-split peaks (3d5/2 and 3d3/2) consistent with bulk CdS (412.1 and 405.3 eV) and surface sulfur vacancies (410.7 and 403.9 eV).53–57 Secondly, the S(2p) spectra for QD-BF4 exhibit peaks corresponding to metal sulfide and metal sulfate (161.6 and 168.7 eV, respectively), indicating that the surface is partially oxidised (Fig. 5).58 After photocatalysis, the proportion of sulfate remains approximately the same for QD-BF4 alone (32.7 and 30.3% before and after photocatalysis, respectively), but decreases significantly in the presence of Co (17.9% and 10.5% after 12 h and 107 h, respectively; Table 2).
0.47) and remained so during photocatalysis (Table 2). The Cd(3d) peaks of QD-OA were broadened compared with those of QD-BF4 (Fig. S10 and S11†), and could be fit to two spin-split peaks (3d5/2 and 3d3/2) consistent with bulk CdS (412.1 and 405.3 eV) and surface sulfur vacancies (410.7 and 403.9 eV).53–57 Secondly, the S(2p) spectra for QD-BF4 exhibit peaks corresponding to metal sulfide and metal sulfate (161.6 and 168.7 eV, respectively), indicating that the surface is partially oxidised (Fig. 5).58 After photocatalysis, the proportion of sulfate remains approximately the same for QD-BF4 alone (32.7 and 30.3% before and after photocatalysis, respectively), but decreases significantly in the presence of Co (17.9% and 10.5% after 12 h and 107 h, respectively; Table 2).
| Condition | Cd/at% | S/at% | Sulfide/% of S | Sulfate/% of S |
|---|---|---|---|---|
| QD-OA | 63.1 ± 0.2 | 36.9 ± 0.9 | 100 ± 0 | — |
| QD-BF4 | 53.7 ± 0.2 | 46.3 ± 0.2 | 67.3 ± 2.3 | 32.7 ± 2.3 |
| QD-BF4, 12 h | 51.8 ± 0.8 | 48.1 ± 0.8 | 69.7 ± 2.6 | 30.3 ± 2.6 |
| QD-BF4/CoCl2, 12 h | 52.8 ± 1.9 | 47.2 ± 1.9 | 82.1 ± 4.6 | 17.9 ± 4.6 |
| QD-BF4/CoCl2, 107 h | 54.1 ± 1.2 | 45.9 ± 1.2 | 89.5 ± 2.6 | 10.5 ± 2.6 |
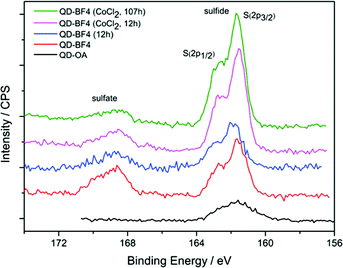 | ||
| Fig. 5 XPS spectra of S(2p) from QD-OA and QD-BF4 particles and QD-BF4 after photocatalysis with and without CoCl2 as a co-catalyst. | ||
Since sulfate is the expected product of both photocorrosion of CdS and oxidation of the electron donor, SO32−,49 it is difficult to determine the origin of these signals. However, the change in stoichiometry and appearance of sulfate during ligand stripping is consistent with etching and the observed change in particle size. Since the subsequent loss of sulfate during photocatalysis is only observed in samples containing Co, it may either be a result of the Co incorporation (e.g. changes in the surface due to Co binding) or be indicative of the nature of the mechanism of activity enhancement (e.g. suppression of photocorrosion).
The activity-enhancing role of the cobalt in this system is not entirely clear. Since no Cd was detected in the supernatant of the centrifuged photocatalysis reaction solution, it is unlikely that a significant proportion of the Co is integrated within the CdS particles by cation exchange. Although we cannot rule out that Co may be incorporated into interstitial Cd vacancies, the primary site for Co attachment is likely to be at the surface, consistent with previous studies of the photooxidative behaviour of CdS in the presence of Co ions,59 and the sulfate displacement observed by XPS. The Co may either be deposited on the surface as an active catalyst species (e.g. CoxSy or CoxOy)47,60 or, alternatively, be adsorbed to the surface as Co ions (e.g. at surface hydroxyl or SOx2− sites), behaving as charge-trapping sites to increase charge-separation and/or promote catalysis on the CdS surface.61
Since the required loading of Co for optimal activity is only two Co ions per QD, the formation of appreciably large domains of an active catalyst species on individual particles is unlikely. However, since the particles are agglomerated, one or more regions of such species might form on the surfaces of particle agglomerates. In this case, there may be many individual QDs with no Co physically attached but which form a conductive network and act as antennae, generating and conducting electrons towards remote active catalyst sites. This mechanism may be one reason why increasing the loading of Co does not increase the activity, since the surface area of the agglomerates is lower than of the individual particles. A corollary of this antenna hypothesis is that agglomeration of the QDs is in fact beneficial for H2 production activity by facilitating charge separation and retarding recombination, as has been shown for H2 production on dye-sensitised, platinised TiO2 nanoparticle powders.62 Since agglomeration has also been reported with the highly active CdTe/CoCl2 (microsphere) and CdSe/Ni(DHLA)2 systems at pH 4–5,25,28 we view this mechanism as an important consideration.
Based on our analysis of the active photocatalyst, we propose that the primary detrimental role of MPA in the QD-MPA/Co system is as a physical barrier. Since thiols do not dissociate from the particles as readily at pH 7 compared to pH 5 and below,35,36 few surface sites on QD-MPA become available during the photoreaction, restricting incorporation of Co onto the particles and limiting substrate/product diffusion to and from the QD surface. The presence of MPA also prevents the particles from aggregating, possibly hindering efficient charge-separation. Finally, we avoid sequestration of Co by MPA, a secondary factor limiting the activity of thiol-capped QD systems.27
Conclusions
We have established ligand stripping as a facile and highly effective method to activate QDs in photocatalytic schemes, which allowed us to construct a QD/Co hybrid system with an unprecedented photoactivity in pH neutral solution. The active system consists of aggregates of stripped – and therefore surface-exposed – CdS particles with an attached cobalt species to enhance catalytic turnover. The photocatalyst system is active for several days, with the buffering ability of the electron donor as the main limiting factor. We anticipate that this approach can also be applied to a wide range of QDs, catalysts and redox transformations.Acknowledgements
We gratefully acknowledge the following funding sources: The Marshall Aid Commemoration Commission (CMC), the Advanced Institute for Materials Research-Cambridge Joint Research Centre (KLO), the Ernest Oppenheimer Fund, Cambridge (BCMM), and the EPSRC (EP/H00338X/2; ER). We would like to thank Dr Fezile Lakadamyali for providing CoP and Dr Anna Reynal-Verdù and Dr Mahmoud Ardakani at Imperial College London for assistance with collecting TEM images of the particles after photocatalysis. We also acknowledge helpful discussions with Dr Moritz F. Kuehnel and Dr Micaela Crespo Quesada.Notes and references
- T. Faunce, S. Styring, M. R. Wasielewski, G. W. Brudvig, A. W. Rutherford, J. Messinger, A. F. Lee, C. L. Hill, H. deGroot, M. Fontecave, D. R. MacFarlane, B. Hankamer, D. G. Nocera, D. M. Tiede, H. Dau, W. Hillier, L. Wang and R. Amal, Energy Environ. Sci., 2013, 6, 1074–1076 Search PubMed.
- J. R. McKone, N. S. Lewis and H. B. Gray, Chem. Mater., 2014, 26, 407–414 CrossRef CAS.
- Y. Tachibana, L. Vayssieres and J. R. Durrant, Nat. Photonics, 2012, 6, 511–518 CrossRef CAS.
- K. Maeda, M. Higashi, D. Lu, R. Abe and K. Domen, J. Am. Chem. Soc., 2010, 132, 5858–5868 CrossRef CAS PubMed.
- C. A. Caputo, M. A. Gross, V. W. Lau, C. Cavazza, B. V. Lotsch and E. Reisner, Angew. Chem., Int. Ed., 2014, 53, 11538–11542 CrossRef CAS PubMed.
- M. A. Gross, A. Reynal, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2014, 136, 356–366 CrossRef CAS PubMed.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655–2661 CrossRef CAS.
- M. B. Wilker, K. J. Schnitzenbaumer and G. Dukovic, Isr. J. Chem., 2012, 52, 1002–1015 CrossRef CAS PubMed.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS.
- J. Sabaté, S. Cervera-March, R. Simarro and J. Giménez, Int. J. Hydrogen Energy, 1990, 15, 115–124 CrossRef.
- N. Bühler, K. Meier and J. F. Reber, J. Phys. Chem., 1984, 88, 3261–3268 CrossRef.
- J. F. Reber and M. Rusek, J. Phys. Chem., 1986, 90, 824–834 CrossRef CAS.
- K. Kalyanasundaram, E. Borgarello, D. Duonghong and M. Grätzel, Angew. Chem., Int. Ed., 1981, 20, 987–988 CrossRef.
- W. Zhang, Y. Wang, Z. Wang, Z. Zhong and R. Xu, Chem. Commun., 2010, 46, 7631–7633 RSC.
- L. Huang, X. Wang, J. Yang, G. Liu, J. Han and C. Li, J. Phys. Chem. C, 2013, 117, 11584–11591 CrossRef CAS.
- L. Huang, J. Yang, X. Wang, J. Han, H. Han and C. Li, Phys. Chem. Chem. Phys., 2013, 15, 553–560 RSC.
- K. A. Brown, S. Dayal, X. Ai, G. Rumbles and P. W. King, J. Am. Chem. Soc., 2010, 132, 9672–9680 CrossRef CAS PubMed.
- K. A. Brown, M. B. Wilker, M. Boehm, G. Dukovic and P. W. King, J. Am. Chem. Soc., 2012, 134, 5627–5636 CrossRef CAS PubMed.
- M. B. Wilker, K. E. Shinopoulos, K. A. Brown, D. W. Mulder, P. W. King and G. Dukovic, J. Am. Chem. Soc., 2014, 136, 4316–4324 CrossRef CAS PubMed.
- B. L. Greene, C. A. Joseph, M. J. Maroney and R. B. Dyer, J. Am. Chem. Soc., 2012, 134, 11108–11111 CrossRef CAS PubMed.
- C.-B. Li, Z.-J. Li, S. Yu, G.-X. Wang, F. Wang, Q.-Y. Meng, B. Chen, K. Feng, C.-H. Tung and L.-Z. Wu, Energy Environ. Sci., 2013, 6, 2597–2602 Search PubMed.
- F. Wang, W.-J. Liang, J.-X. Jian, C.-B. Li, B. Chen, C.-H. Tung and L.-Z. Wu, Angew. Chem., Int. Ed., 2013, 52, 8134–8138 CrossRef CAS PubMed.
- F. Wang, W.-G. Wang, X.-J. Wang, H.-Y. Wang, C.-H. Tung and L.-Z. Wu, Angew. Chem., Int. Ed., 2011, 50, 3193–3197 CrossRef CAS PubMed.
- Z.-J. Li, X.-B. Li, J.-J. Wang, S. Yu, C.-B. Li, C.-H. Tung and L.-Z. Wu, Energy Environ. Sci., 2013, 6, 465–469 Search PubMed.
- C. Gimbert-Suriñach, J. Albero, T. Stoll, J. Fortage, M.-N. Collomb, A. Deronzier, E. Palomares and A. Llobet, J. Am. Chem. Soc., 2014, 136, 7655–7661 CrossRef PubMed.
- A. Das, Z. Han, M. G. Haghighi and R. Eisenberg, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 16716–16723 CrossRef CAS PubMed.
- Z. Han, F. Qiu, R. Eisenberg, P. L. Holland and T. D. Krauss, Science, 2012, 338, 1321–1324 CrossRef CAS PubMed.
- Z.-J. Li, X.-B. Fan, X.-B. Li, J.-X. Li, C. Ye, J.-J. Wang, S. Yu, C.-B. Li, Y.-J. Gao, Q.-Y. Meng, C.-H. Tung and L.-Z. Wu, J. Am. Chem. Soc., 2014, 136, 8261–8268 CrossRef CAS PubMed.
- T. Simon, N. Bouchonville, M. J. Berr, A. Vaneski, A. Adrović, D. Volbers, R. Wyrwich, M. Döblinger, A. S. Susha, A. L. Rogach, F. Jäckel, J. K. Stolarczyk and J. Feldmann, Nat. Mater., 2014, 13, 1013–1018 CrossRef CAS PubMed.
- F. Lakadamyali, M. Kato, N. M. Muresan and E. Reisner, Angew. Chem., Int. Ed., 2012, 51, 9381–9384 CrossRef CAS PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- M. A. Holmes, T. K. Townsend and F. E. Osterloh, Chem. Commun., 2012, 48, 371–373 RSC.
- K. P. Acharya, R. S. Khnayzer, T. O’Connor, G. Diederich, M. Kirsanova, A. Klinkova, D. Roth, E. Kinder, M. Imboden and M. Zamkov, Nano Lett., 2011, 11, 2919–2926 CrossRef CAS PubMed.
- J. Aldana, N. Lavelle, Y. Wang and X. Peng, J. Am. Chem. Soc., 2005, 127, 2496–2504 CrossRef CAS PubMed.
- M. J. Mulvihill, S. E. Habas, I. Jen-La Plante, J. Wan and T. Mokari, Chem. Mater., 2010, 22, 5251–5257 CrossRef CAS.
- F. Lakadamyali and E. Reisner, Chem. Commun., 2011, 47, 1695–1697 RSC.
- E. L. Rosen, R. Buonsanti, A. Llordes, A. M. Sawvel, D. J. Milliron and B. A. Helms, Angew. Chem., Int. Ed., 2012, 51, 684–689 CrossRef CAS PubMed.
- W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854–2860 CrossRef CAS.
- J. Liu, X. Yang, K. Wang, D. Wang and P. Zhang, Chem. Commun., 2009, 6080–6082 RSC.
- F. Lakadamyali, A. Reynal, M. Kato, J. R. Durrant and E. Reisner, Chem.–Eur. J., 2012, 18, 15464–15475 CrossRef CAS PubMed.
- J. Willkomm, N. M. Muresan and E. Reisner, Chem. Sci., 2015, 6, 2727–2736 RSC.
- B. S. Veldkamp, W.-S. Han, S. M. Dyar, S. W. Eaton, M. A. Ratner and M. R. Wasielewski, Energy Environ. Sci., 2013, 6, 1917–1928 Search PubMed.
- D. W. Wakerley and E. Reisner, Phys. Chem. Chem. Phys., 2014, 16, 5739–5746 RSC.
- N. M. Muresan, J. Willkomm, D. Mersch, Y. Vaynzof and E. Reisner, Angew. Chem., Int. Ed., 2012, 51, 12749–12753 CrossRef CAS PubMed.
- L. A. Berben and J. C. Peters, Chem. Commun., 2010, 46, 398–400 RSC.
- S. Cobo, J. Heidkamp, P.-A. Jacques, J. Fize, V. Fourmond, L. Guetaz, B. Jousselme, V. Ivanova, H. Dau, S. Palacin, M. Fontecave and V. Artero, Nat. Mater., 2012, 11, 802–807 CrossRef CAS PubMed.
- T. M. McCormick, B. D. Calitree, A. Orchard, N. D. Kraut, F. V. Bright, M. R. Detty and R. Eisenberg, J. Am. Chem. Soc., 2010, 132, 15480–15483 CrossRef CAS PubMed.
- D. Meissner, R. Memming and B. Kastening, J. Phys. Chem., 1988, 92, 3476–3483 CrossRef CAS.
- P. Neta and R. E. Huie, Environ. Health Perspect., 1985, 64, 209–217 CrossRef CAS PubMed.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- A. Reynal, E. Pastor, M. A. Gross, S. Selim, E. Reisner and J. R. Durrant, Chem. Sci., 2015 10.1039/c5sc01349f.
- Y.-M. Fang, J. Song, R.-J. Zheng, Y.-M. Zeng and J.-J. Sun, J. Phys. Chem. C, 2011, 115, 9117–9121 CrossRef CAS.
- A. Veamatahau, B. Jiang, T. Seifert, S. Makuta, K. Latham, M. Kanehara, T. Teranishi and Y. Tachibana, Phys. Chem. Chem. Phys., 2015, 17, 2850–2858 RSC.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, Handbook of X-ray Photoelectron Spectroscopy, Physical Electronics Inc., Minnesota, USA, 1992 Search PubMed.
- H. L. Lee, A. M. Issam, M. Belmahi, M. B. Assouar, H. Rinnert and M. Alnot, J. Nanomater., 2009, 914501 Search PubMed.
- S. Rengaraj, S. Venkataraj, S. H. Jee, Y. Kim, C.-w. Tai, E. Repo, A. Koistinen, A. Ferancova and M. Sillanpää, Langmuir, 2011, 27, 352–358 CrossRef CAS PubMed.
- J. I. Kim, J. Kim, J. Lee, D.-R. Jung, H. Kim, H. Choi, S. Lee, S. Byun, S. Kang and B. Park, Nanoscale Res. Lett., 2012, 7, 482–488 CrossRef PubMed.
- Y. H. Hsieh, C. P. Huang and A. P. Davis, Chemosphere, 1992, 24, 281–290 CrossRef CAS.
- C.-Y. Lin, D. Mersch, D. A. Jefferson and E. Reisner, Chem. Sci., 2014, 5, 4906–4913 RSC.
- S. R. Pendlebury, X. Wang, F. Le Formal, M. Cornuz, A. Kafizas, S. D. Tilley, M. Grätzel and J. R. Durrant, J. Am. Chem. Soc., 2014, 136, 9854–9857 CrossRef CAS PubMed.
- Y. Park, W. Kim, D. Monllor-Satoca, T. Tachikawa, T. Majima and W. Choi, J. Phys. Chem. Lett., 2013, 4, 189–194 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures, tables, and experimental details. See DOI: 10.1039/c5ta04136h. Additional data related to this publication is available at the University of Cambridge data repository (https://www.repository.cam.ac.uk/handle/1810/248732). |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |