Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective bifunctional iminophosphorane catalyzed sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters†
Jinchao
Yang
,
Alistair J. M.
Farley
and
Darren J.
Dixon
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, UK. E-mail: Darren.dixon@chem.ox.ac.uk
First published on 14th September 2016
Abstract
The highly enantioselective sulfa-Michael addition of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters catalyzed by a bifunctional iminophosphorane (BIMP) organocatalyst is described. The low acidity of the alkyl thiol pro-nucleophiles is overcome by the high Brønsted basicity of the catalyst and the chiral scaffold/thiourea hydrogen-bond donor moiety provides the required enantiofacial discrimination in the addition step. The reaction is broad in scope with respect to the alkyl thiol and β-substituent of the α,β-unsaturated ester, affords sulfa-Michael adducts in excellent yields (up to >99%) and enantioselectivity (up to 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er) and can operate down to 1 mol% catalyst loading.
3 er) and can operate down to 1 mol% catalyst loading.
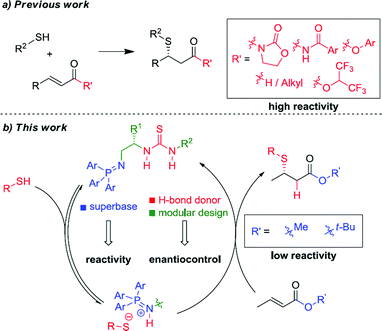
Unactivated β-substituted-α,β-unsaturated esters, such as methyl crotonate, methyl cinnamate and their homologues, are a class of low reactivity electrophiles that offer a wealth of untapped potential in the field of enantioselective organocatalysis.1 To date, these esters have remained a persistent challenge as Michael acceptors in asymmetric catalysis using both metal-rich and metal-free catalyst systems, largely due to their low inherent electrophilicity2 and low propensity for catalyst activation and enantioface discrimination.3,4 They are commercial and cheap, or are readily prepared by a variety of standard methods and are stable. In contrast to commonly used (reactive) Michael acceptors such as nitroolefins, they lie at the bottom of the Mayr electrophile reactivity (E) scale,5,6 and unlike enal and enone Michael acceptors they cannot be activated through iminium ion formation with chiral amine catalysts.7 Related literature examples employ activated carboxylic derivatives8 such as N-enoyl imides, N-enoyl oxazolidinones, perfluorinated alkyl esters, thioamides, N-enoyl pyrroles and, most recently, aryl esters.9 Alternatively, activating substituents at the α- or β-positions can also be used to gain reactivity and/or stereoselectivity. To illustrate the case in point, to date there has not been a single report of a highly enantioselective addition of a pro-nucleophilic reagent [a carbon-centered (C–H) or heteroatom-centered (X–H) acid] to unactivated alkyl cinnamate or crotonate esters under organocatalytic conditions.10 Effectively, these cheap chemical feedstocks are out of reach of existing chiral organocatalysts and accordingly are a very attractive ‘simple’ target class of electrophiles for new enantioselective organocatalytic reaction development (Fig. 1).
 | ||
| Fig. 1 Bifunctional Brønsted base/H-bond donor organocatalytic SMA to α,β-unsaturated ester derivatives. | ||
A proven strategy to overcome low substrate electrophilicity in base-catalyzed polar addition reactions is to increase the concentration of the nucleophilic conjugate base in the pot – and therefore the rate of the nucleophilic addition reaction – by enhancing the Brønsted basicity of the catalyst relative to tertiary amine catalysts.11–13 To this end, we disclosed that bifunctional iminophosphorane (BIMP) catalysts, containing a novel organosuperbase were highly efficacious in the first general enantioselective organocatalytic ketimine nitro-Mannich reaction.12b,d Likewise, very recently, high catalyst performance (in terms of reactivity and enantioselectivity) with a second generation BIMP catalyst was also witnessed in the first organocatalytic conjugate addition of alkyl thiols to unactivated α-substituted acrylate esters (such as methyl methacrylate).12e In both of these transformations an organosuperbase was demonstrated to be essential for reactivity.
We speculated that the reluctance of unactivated β-substituted-α,β-unsaturated esters to undergo organocatalytic Michael addition reactions could be overcome using our BIMP catalyst family. To exemplify this we chose the sulfa-Michael addition (SMA) of alkyl thiols as this is a reaction of central importance for the asymmetric construction of chiral sulfides possessing a stereogenic centre at the β-carbon and no organocatalytic enantioselective version has previously been reported.14,15 We reasoned that the high Brønsted basicity of our BIMP catalysts could activate the high pKa alkyl thiol pro-nucleophile (pKa(DMSO) = 17 for n-BuSH)16,17 and the modular design of the catalyst family, through its variable backbone scaffold, hydrogen-bond donor group and iminophosphorane superbase would expedite optimal catalyst identification. Herein, and as part of our research program towards the development of novel asymmetric reactions with challenging electrophile/pro-nucleophile combinations, we wish to report our investigations leading to the highly enantioselective SMA reaction of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters.
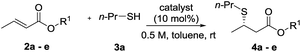
We chose commercially available methyl crotonate (2a) and 1-propanethiol (3a) as our model system and investigated reactivity using first generation BIMP catalyst 1a (Table 1, entry 1). In toluene, at room temperature using 10 mol% catalyst we were delighted to observe an exceptional reactivity profile; β-mercaptoester product 4a was afforded in near quantitative yield after only 2 hours with low but significant enantiocontrol (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 er).18 With good reactivity established we next investigated the performance of a small library of second generation BIMP catalysts featuring variations around the amide–thiourea motif that we recently reported12e (Table 1, entries 2–6). The modular design of our BIMP catalysts allowed rapid library generation and our attention focussed on the amide–thiourea moiety as the H-bond donor group and the tris-(4-methoxyphenylphosphine) derived iminophosphorane as the Brønsted basic group (Fig. 2).
45 er).18 With good reactivity established we next investigated the performance of a small library of second generation BIMP catalysts featuring variations around the amide–thiourea motif that we recently reported12e (Table 1, entries 2–6). The modular design of our BIMP catalysts allowed rapid library generation and our attention focussed on the amide–thiourea moiety as the H-bond donor group and the tris-(4-methoxyphenylphosphine) derived iminophosphorane as the Brønsted basic group (Fig. 2).
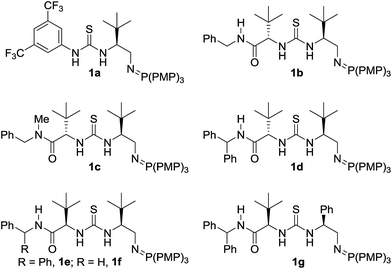 | ||
| Fig. 2 Bifunctional iminophosphorane (BIMP) organocatalysts used in the optimization of the SMA reaction. PMP = p-methoxyphenyl. | ||
| Entry | Cat. | R1 | Product | Time (h) | Yieldb (%) | erc |
|---|---|---|---|---|---|---|
| a Reactions were carried out with 0.20 mmol of 2 and 0.60 mmol of 3a. b Isolated yield. c Determined by HPLC analysis on a chiral stationary phase. d Reaction performed on 0.10 mmol scale of 2a. e Reaction performed at 0 °C. f Reaction performed at 0 °C in Et2O. g Reaction performed at −15 °C in Et2O. | ||||||
| 1 | 1a | Me | 4a | 2 | 94 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 2 | 1b | Me | 4a | 2 | 98 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 45 |
| 3 | 1c | Me | 4a | 2 | 94 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 4 | 1d | Me | 4a | 2 | 93 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
| 5 | 1e | Me | 4a | 2 | >99 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 6 | 1f | Me | 4a | 2 | 97 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 7d | 1g | Me | 4a | 3 | >99 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 8 | 1g | Et | 4b | 3 | 95 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 9 | 1g | i-Pr | 4c | 3 | >99 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 10 | 1g | Bn | 4d | 3 | >99 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 11d | 1g | t-Bu | 4e | 8 | 94 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 12d,e | 1g | t-Bu | 4e | 8 | 95 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 13f | 1g | t-Bu | 4e | 24 | 94 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 14g | 1g | t-Bu | 4e | 72 | 94 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Catalysts 1b–d possessing a thiourea constructed from two (S)-configured tert-leucine derived residues, the tris-(4-methoxyphenylphosphine)-derived iminophosphorane and a variable terminal amide group gave poor enantioselectivity in all cases (Table 1, entries 2, 3, and 4). When catalyst 1e – the diastereomer of 1d – was trialled however, a significant boost to the enantioselectivity was witnessed (Table 1, entry 5, 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 er).19
25 er).19
A comparison with an analogous catalyst possessing a phenylglycine and a tert-leucine residue (1g) resulted in a slight improvement to the enantioselectivity (Table 1, entry 7, 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 er). At this stage, the effect of varying the ester group of the crotonate on the enantioselectivity in the SMA was investigated. A range of simple, commercial or readily synthesized alkyl crotonate esters were trialled and a correlation between the size of the ester group and the enantioselectivity was observed – pleasingly tert-butyl crotonate (2e) afforded the product 4e in 92
19 er). At this stage, the effect of varying the ester group of the crotonate on the enantioselectivity in the SMA was investigated. A range of simple, commercial or readily synthesized alkyl crotonate esters were trialled and a correlation between the size of the ester group and the enantioselectivity was observed – pleasingly tert-butyl crotonate (2e) afforded the product 4e in 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er albeit in a slightly increased reaction time of 8 h (Table 1, entry 11). A reoptimization of the reaction conditions to 0.5 M in Et2O at 0 °C (Table 1, entries 12 & 13 and ESI†) resulted in a significant boost to the enantioselectivity (96
8 er albeit in a slightly increased reaction time of 8 h (Table 1, entry 11). A reoptimization of the reaction conditions to 0.5 M in Et2O at 0 °C (Table 1, entries 12 & 13 and ESI†) resulted in a significant boost to the enantioselectivity (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 er) and cooling the reaction temperature further to −15 °C afforded β-mercaptoester 4e in 94% yield and 97
4 er) and cooling the reaction temperature further to −15 °C afforded β-mercaptoester 4e in 94% yield and 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er (Table 1, entry 14).
3 er (Table 1, entry 14).
With optimized reaction conditions established, the scope of the transformation with respect to the thiol pro-nucleophile and the α,β-unsaturated ester was investigated (Fig. 3). Minimal variation to the enantioselectivity was observed across a good range of linear (propyl to decyl) or branched (cyclic and acyclic) alkyl mercaptans. The reaction with 4-methoxybenzyl mercaptan was also well-tolerated and afforded the β-mercaptoester 4m in 99% yield and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er at −15 °C.
5 er at −15 °C.
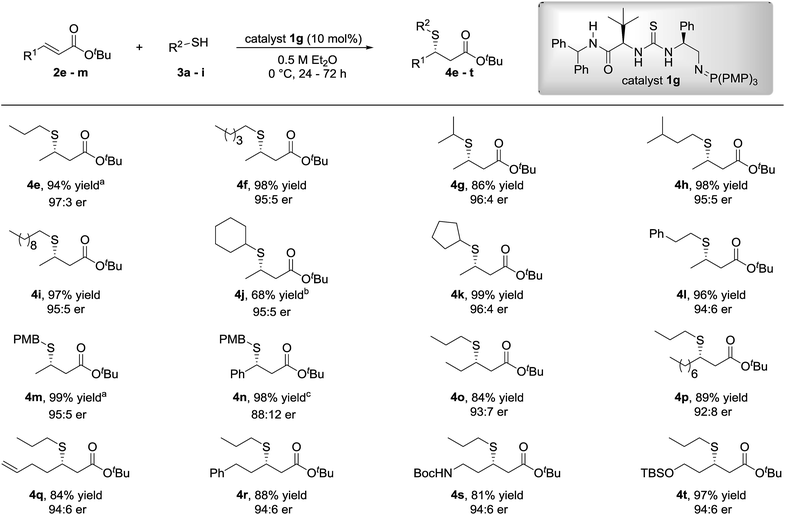 | ||
| Fig. 3 Scope of the SMA of alkyl thiols to β-substituted-α,β-unsaturated esters. Reactions were carried out with 0.20 mmol 2 and 0.60 mmol 3. Yields are isolated yields and enantiomeric ratios were determined by HPLC analysis or GC analysis on a chiral stationary phase. aThe reaction was performed at −15 °C. bThe reaction was quenched after 96 h. cAbsolute configuration of 4n determined by chemical correlation (see ESI†). | ||
Following investigation into the scope of the reaction with respect to the alkyl thiol, variation to the β-substituent of the α,β-unsaturated ester was subsequently examined using 1-propanethiol or 4-methoxybenzyl mercaptan as the sulfur-centred pro-nucleophile. We were pleased to observe that the excellent reactivity and selectivities were maintained when tert-butyl cinnamate, bearing a phenyl group at the β-position, was used as the electrophile to afford the desired β-mercaptoester 4n in 98% yield and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er.
12 er.
Similarly, excellent yields of the β-mercaptoesters 4o–r were obtained from the corresponding primary alkyl β-substituted-α,β-unsaturated esters with very good levels of enantiocontrol. β-Mercaptoesters 4s and 4t containing a terminal N-Boc protected amine and TBS protected hydroxyl group respectively were also synthesized in good to excellent yields and excellent enantiomeric ratios.
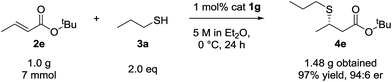
Although the scope of the reaction was performed with 10 mol% catalyst loading, we were keen to demonstrate lower loadings were viable. Accordingly, and after reoptimization of the reaction conditions, to 5 M in Et2O at 0 °C, we were pleased to find β-mercaptoester 4e was afforded in near quantitative yield and 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 er on 7 mmol scale of tert-butyl crotonate (2e) using 1 mol% catalyst 1g (Scheme 1).
6 er on 7 mmol scale of tert-butyl crotonate (2e) using 1 mol% catalyst 1g (Scheme 1).
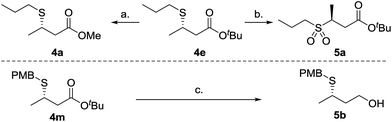
To demonstrate synthetic utility of the β-mercaptoester products a selection of standard chemical transformations were carried out (Scheme 2). Thus β-mercaptoester 4e (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er) was transesterified to the methyl ester 4a in a two step process; initial acidic cleavage of the tert-butyl ester and subsequent methyl ester formation under acidic conditions afforded 4a in 78% yield without compromising stereochemical integrity. Oxidation of 4e afforded sulfone 5a without any observable racemization in near quantitative yield. Finally, β-mercaptoester 4m was reduced to the alcohol in excellent yield, without appreciable loss of enantiopurity.20
5 er) was transesterified to the methyl ester 4a in a two step process; initial acidic cleavage of the tert-butyl ester and subsequent methyl ester formation under acidic conditions afforded 4a in 78% yield without compromising stereochemical integrity. Oxidation of 4e afforded sulfone 5a without any observable racemization in near quantitative yield. Finally, β-mercaptoester 4m was reduced to the alcohol in excellent yield, without appreciable loss of enantiopurity.20
In summary, we have developed the first organocatalytic enantioselective SMA of alkyl thiols to unactivated β-substituted-α,β-unsaturated esters. Impressive reactivity and excellent levels of enantioselectivities were achieved across a range of linear, branched, cyclic alkyl and benzylic thiols, in SMA reactions to various β-substituted-α,β-unsaturated esters using a novel bifunctional iminophosphorane catalyst. This work demonstrates that the high reactivity of the BIMP catalysts enables low reactivity electrophiles such as β-substituted-α,β-unsaturated esters to undergo highly enantioselective conjugate addition reactions for the first time and thus represents a significant advance in the field. Work to uncover further capabilities of the BIMP catalyst family is ongoing in our laboratories and the results will be disclosed in due course.
Acknowledgements
We thank the EPSRC (Studentship and Doctoral Training Grant [EP/M50659X/1] to A. J. M. F.), AstraZeneca (Studentship to A. J. M. F.), the SCI (Postgraduate Scholarship to A. J. M. F.) and the China Scholarship Council-University of Oxford Scholarship (Studentship to J. Y.) for funding.Notes and references
- For reviews on organocatalysis, see: (a) P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed; (b) A. Berkessel and H. Gröger, Asymmetric Organocatalysis, Wiley VCH, Weinheim, 2005 Search PubMed; (c) B. List and J. W. Yang, Science, 2006, 313, 1584 CrossRef CAS PubMed; (d) A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638 CrossRef CAS PubMed; (e) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC; (f) C. Palomo, M. Oiarbide and R. López, Chem. Soc. Rev., 2009, 38, 632 RSC.
- (a) S. Matsunaga, T. Kinoshita, S. Okada, S. Harada and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 7559 CrossRef CAS PubMed; (b) F. López, S. R. Harutyunyan, A. Meetsma, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2005, 44, 2752 CrossRef PubMed; (c) C. Pubill-Ulldemolins, A. Bonet, C. Bo, H. Gulyás and E. Fernández, Chem.–Eur. J., 2012, 18, 1121 CrossRef CAS PubMed.
- K. Tomioka and Y. Nagaoka, Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfatlz and H. Yamamoto, Springer, New York, 1999, vol. 3 Search PubMed.
- For examples of enantioselective organocatalytic cycloaddition reactions using crotonate and cinnamate esters, see: (a) B. Mathieu, L. de Fays and L. Ghosez, Tetrahedron Lett., 2000, 41, 9561 CrossRef CAS; (b) D. H. Ryu, T. W. Lee and E. J. Corey, J. Am. Chem. Soc., 2002, 124, 9992 CrossRef CAS PubMed; (c) T. Gatzenmeier, M. van Gemmeren, Y. Xie, D. Höfler, M. Leutzsch and B. List, Science, 2016, 351, 949 CrossRef CAS PubMed.
- The electrophile-specific reactivity parameters E of trans-β-nitrostyrenes have been determined to be −15 < E < −12: I. Zenz and H. Mayr, J. Org. Chem., 2011, 76, 9370 CrossRef CAS PubMed.
- Preliminary studies indicate the electrophile-specific reactivity parameters E of ethyl cinnamate and ethyl crotonate are in the range of −25 < E < −23: H. Mayr, personal communication.
- For a review on iminium catalysis, see: A. Erkkilä, I. Majander and P. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef PubMed.
- For reviews on organocatalytic conjugate addition reactions, see: (a) J. L. Vicario, D. Badía, L. Carrillo and E. Reyes, Organocatalytic Enantioselective Conjugate Addition Reactions, Royal Society of Chemistry, Cambridge, 2010 Search PubMed; (b) S. B. Tsogoeva, Eur. J. Org. Chem., 2007, 1701 CrossRef CAS.
- The organocatalytic enantioselective conjugate addition of benzyl mercaptan to activated aryl crotonate esters was reported with a N-heterocyclic carbene catalyst: P. Yuan, S. Meng, J. Chen and Y. Huang, Synlett, 2016, 27, 1068 CrossRef CAS.
- To the best of our knowledge, only a single isolated example of a moderately enantioselective organocatalytic Michael addition to ethyl crotonate has been reported, see: X. Dong, X. Fang and C.-J. Wang, Org. Lett., 2011, 13, 4426 CrossRef CAS PubMed.
- For reviews on Brønsted base H-bond donor bifunctional organocatalysts, see: (a) Y. Takemoto, Org. Biomol. Chem., 2005, 3, 4299 RSC; (b) S. J. Connon, Chem. Commun., 2008, 2499 RSC; (c) T. Marcelli and H. Hiemstra, Synthesis, 2010, 1229 CrossRef CAS.
- For the use of chiral organosuperbase catalysts to enhance reactivity, see: (a) J. S. Bandar and T. H. Lambert, J. Am. Chem. Soc., 2012, 134, 5552 CrossRef CAS PubMed; (b) M. G. Núñez, A. J. M. Farley and D. J. Dixon, J. Am. Chem. Soc., 2013, 135, 16348 CrossRef PubMed; (c) T. Takeda and M. Terada, J. Am. Chem. Soc., 2013, 135, 15306 CrossRef CAS PubMed; (d) A. M. Goldys, M. G. Núñez and D. J. Dixon, Org. Lett., 2014, 16, 6294 CrossRef CAS PubMed; (e) A. J. M. Farley, C. Sandford and D. J. Dixon, J. Am. Chem. Soc., 2015, 137, 15992 CrossRef CAS PubMed; (f) G. P. Robertson, A. J. M. Farley and D. J. Dixon, Synlett, 2016, 27, 21 CAS; (g) M. A. Horwitz, B. P. Zavesky, J. Martinez-Alvarado and J. S. Johnson, Org. Lett., 2016, 18, 36 CrossRef CAS PubMed.
- For the use of organosuperbases in synthesis, see: (a) T. Ishikawa, Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts, Wiley, New York, 2009 Search PubMed; For a review on chiral organosuperbases, see: (b) T. Ishikawa and T. Kumamoto, Synthesis, 2006, 737 CrossRef CAS; (c) D. Leow and C.-H. Tan, Chem.–Asian J., 2009, 4, 488 CrossRef CAS PubMed; (d) D. Leow and C.-H. Tan, Synlett, 2010, 1589 CAS; (e) T. Ishikawa, Chem. Pharm. Bull., 2010, 58, 1555 CrossRef CAS PubMed; (f) X. Fu and C.-H. Tan, Chem. Commun., 2011, 47, 8210 RSC; (g) P. Selig, Synthesis, 2013, 45, 703 CrossRef CAS; (h) H. Krawczyk, M. Dzięgielewski, D. Deredas, A. Albrecht and Ł. Albrecht, Chem.–Eur. J., 2015, 21, 10268 CrossRef CAS PubMed. For selected examples, see: (i) E. J. Corey and M. J. Grogan, Org. Lett., 1999, 1, 157 CrossRef CAS PubMed; (j) T. Ishikawa, Y. Araki, T. Kumamoto, H. Seki, K. Fukuda and T. Isobe, Chem. Commun., 2001, 245 RSC; (k) B. M. Nugent, R. A. Yoder and J. N. Johnston, J. Am. Chem. Soc., 2004, 126, 3418 CrossRef CAS PubMed; (l) M. Terada, H. Ube and Y. Yaguchi, J. Am. Chem. Soc., 2006, 128, 1454 CrossRef CAS PubMed; (m) D. Uraguchi, S. Sakaki and T. Ooi, J. Am. Chem. Soc., 2007, 129, 12392 CrossRef CAS PubMed; (n) T. A. Davis, J. C. Wilt and J. N. Johnston, J. Am. Chem. Soc., 2010, 132, 2880 CrossRef CAS PubMed; (o) Y. Sohtome, B. Shin, N. Horitsugi, R. Takagi, K. Noguchi and K. Nagasawa, Angew. Chem., Int. Ed., 2010, 49, 7299 CrossRef CAS PubMed; (p) T. A. Davis and J. N. Johnston, Chem. Sci., 2011, 2, 1076 RSC; (q) D. Uraguchi, K. Yoshioka, Y. Ueki and T. Ooi, J. Am. Chem. Soc., 2012, 134, 19370 CrossRef CAS PubMed; (r) M. T. Corbett, D. Uraguchi, T. Ooi and J. S. Johnson, Angew. Chem., Int. Ed., 2012, 51, 4685 CrossRef CAS PubMed; (s) T. Misaki, N. Jin, K. Kawano and T. Sugimura, Chem. Lett., 2012, 41, 1675 CrossRef CAS; (t) T. E. Shubina, M. Freund, S. Schenker, T. Clark and S. B. Tsogoeva, Beilstein J. Org. Chem., 2012, 8, 1485 CrossRef CAS PubMed; (u) J. S. Bandar and T. H. Lambert, J. Am. Chem. Soc., 2013, 135, 11799 CrossRef CAS PubMed; (v) J. S. Bandar, A. Barthelme, A. Y. Mazori and T. H. Lambert, Chem. Sci., 2015, 6, 1537 RSC; (w) D. Uraguchi, K. Yamada and T. Ooi, Angew. Chem., Int. Ed., 2015, 54, 9954 CrossRef CAS PubMed; (x) M. Işik, M. Y. Unver and C. Tanyeli, J. Org. Chem., 2015, 80, 828 CrossRef PubMed; (y) X. Gao, J. Han and L. Wang, Org. Lett., 2015, 17, 4596 CrossRef CAS PubMed; (z) J. Chen, S. Meng, L. Wang, H. Tang and Y. Huang, Chem. Sci., 2015, 6, 4184 RSC.
- For a review on asymmetric sulfa-Michael additions, see: (a) D. Enders, K. Lüttgen and A. A. Narine, Synthesis, 2007, 959 CrossRef CAS. For selected examples using metals, see: (b) K. Nishimura, M. Ono, Y. Nagaoka and K. J. Tomioka, J. Am. Chem. Soc., 1997, 119, 12974 CrossRef CAS; (c) S. Kanemasa, Y. Oderaotoshi and E. Wada, J. Am. Chem. Soc., 1999, 121, 8675 CrossRef CAS; (d) K. Nishimura, M. Ono, Y. Nagaoka and K. Tomioka, Angew. Chem., Int. Ed., 2001, 40, 440 CrossRef CAS; (e) Y. Hui, J. Jiang, W. Wang, W. Chen, Y. Cai, L. Lin, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2010, 49, 4290 CrossRef CAS PubMed; (f) S. Bonollo, D. Lanari, F. Pizzo and L. Vaccaro, Org. Lett., 2011, 13, 2150 CrossRef CAS PubMed; (g) T. Kitanosono, M. Sakai, M. Ueno and S. Kobayashi, Org. Biomol. Chem., 2012, 10, 7134 RSC; (h) T. Ogawa, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2012, 51, 8551 CrossRef CAS PubMed.
- For a review on organocatalytic asymmetric SMA reactions, see: (a) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807 CrossRef CAS PubMed. For selected examples, see: (b) H. Hiemstra and H. Wynberg, J. Am. Chem. Soc., 1981, 103, 417 CrossRef CAS; (c) A. Kumar, R. V. Salunkhe, R. A. Rane and S. Y. Dike, J. Chem. Soc., Chem. Commun., 1991, 485 RSC; (d) P. McDaid, Y. Chen and L. Deng, Angew. Chem., Int. Ed., 2002, 41, 338 CrossRef CAS; (e) B.-J. Li, L. Jiang, M. Liu, Y.-C. Chen, L.-S. Ding and Y. Wu, Synlett, 2005, 603 CAS; (f) D. Leow, L. Shishi, S. K. Chittimalla, X. Fu and C.-H. Tan, Angew. Chem., Int. Ed., 2008, 47, 5641 CrossRef CAS PubMed; (g) Y. Liu, B. Sun, B. Wang, M. Wakem and L. Deng, J. Am. Chem. Soc., 2009, 131, 418 CrossRef CAS PubMed; (h) K. L. Kimmel, M. T. Robak and J. A. Ellman, J. Am. Chem. Soc., 2009, 131, 8754 CrossRef CAS PubMed; (i) N. K. Rana, S. Selvakumar and V. K. Singh, J. Org. Chem., 2010, 75, 2089 CrossRef CAS PubMed; (j) L. Dai, S.-X. Wang and F.-E. Chen, Adv. Synth. Catal., 2010, 352, 2137 CrossRef CAS; (k) N. K. Rana and V. K. Singh, Org. Lett., 2011, 13, 6520 CrossRef CAS PubMed; (l) L. Dai, H. Yang and F. Chen, Eur. J. Org. Chem., 2011, 5071 CrossRef CAS; (m) C. Palacio and S. Connon, Chem. Commun., 2012, 48, 2849 RSC; (n) D. Uraguchi, N. Kinoshita, D. Nakashima and T. Ooi, Chem. Sci., 2012, 3, 3161 RSC; (o) L. Dai, H. Yang, J. Niu and F. Chen, Synlett, 2012, 314 CAS; (p) A. C. Breman, J. M. M. Smits, R. de Gelder, J. H. van Maarseveen, S. Ingemann and H. Hiemstra, Synlett, 2012, 23, 2195 CrossRef CAS; (q) X. Fang, J. Li and C.-J. Wang, Org. Lett., 2013, 15, 3448 CrossRef CAS PubMed; (r) R. A. Unhale, N. K. Rana and V. K. Singh, Tetrahedron Lett., 2013, 54, 1911 CrossRef CAS; (s) R. Wang, J. Liu and J. Xu, Adv. Synth. Catal., 2014, 357, 159 CrossRef; (t) J. P. Phelan, E. J. Patel and J. A. Ellman, Angew. Chem., Int. Ed., 2014, 53, 11329 CrossRef CAS PubMed; (u) N. K. Fu, L. Zhang, S. Z. Luo and J. P. Cheng, Org. Lett., 2014, 16, 4626 CrossRef CAS PubMed.
- F. G. Bordwell and D. L. Hughes, J. Org. Chem., 1982, 47, 3224 CrossRef CAS.
- For comparison, the pKa of thiophenol in DMSO is 10.3. See ref. 16.
- Bifunctional cinchonine derived bifunctional thiourea catalysts [890044-38-9] were found to be impotent in this transformation. After 7 days under analogous conditions no addition product 4a was observed by 1H NMR analysis of the crude reaction mixture.
- The reaction with PhSH, 2a and catalyst 1e proceeded with 93% yield and 66
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 er.
34 er. - The PMB thiol can be readily cleaved to afford the free mercaptan, see for example ref. 15g.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, copies of 1H and 13C NMR spectra and HPLC and GC chromatograms. See DOI: 10.1039/c6sc02878k |
| This journal is © The Royal Society of Chemistry 2017 |