Open Access Article
Open Access ArticleGas phase stabiliser-free production of hydrogen peroxide using supported gold–palladium catalysts†
Adeeba
Akram
a,
Simon J.
Freakley
a,
Christian
Reece
a,
Marco
Piccinini
a,
Greg
Shaw
a,
Jennifer K.
Edwards
a,
Frédérique
Desmedt
b,
Pierre
Miquel
b,
Eero
Seuna
b,
David. J.
Willock
a,
Jacob A.
Moulijn
a and
Graham J.
Hutchings
*a
aCardiff Catalysis Institute and School of Chemistry, Main Building, Park Place, Cardiff, CF10 3AT, UK. E-mail: Hutch@cardiff.ac.uk
bSolvay, Rue de Ransbeek, 310, B-1120 Brussels, Belgium
First published on 11th May 2016
Abstract
Hydrogen peroxide synthesis from hydrogen and oxygen in the gas phase is postulated to be a key reaction step in the gas phase epoxidation of propene using gold–titanium silicate catalysts. During this process H2O2 is consumed in a secondary step to oxidise an organic molecule so is typically not observed as a reaction product. We demonstrate that using AuPd nanoparticles, which are known to have high H2O2 synthesis rates in the liquid phase, it is possible to not only oxidise organic molecules in the gas phase but to detect H2O2 for the first time as a reaction product in both a fixed bed reactor and a pulsed Temporal Analysis of Products (TAP) reactor without stabilisers present in the gas feed. This observation opens up possibility of synthesising H2O2 directly using a gas phase reaction.
Introduction
The direct synthesis of hydrogen peroxide (H2O2) from molecular H2 and O2 in the liquid phase is an active area of research in the field of heterogeneous catalysts.1 Catalysts based on Pd and Au–Pd nanoparticles supported on carbon and oxide materials as well as homogeneous gold/palladium systems show interesting properties and have been widely studied in many solvent systems.2–7 The direct combination of H2 and O2 to form H2O2 is carried out with a view to both synthesising viable concentrations of H2O2 and utilising synthesised H2O2 to carry out in situ oxidation reactions, including the epoxidation of propene.One of the major problems associated with the vapour phase oxidation of propene to propene oxide (PO) using molecular oxygen is low reaction selectivity. The formation of the epoxide requires the electrophilic addition of an oxygen intermediate to the carbon–carbon double bond, however, propene can also be easily activated via the formation of allylic species which leads to non-selective oxidation. The addition of H2 to O2 streams as a sacrificial reductant, to synthesise H2O2 or peroxy species in situ, permits the activation of dioxygen at temperatures that are typically much lower than required to activate oxygen alone. Haruta and co-workers were the first to demonstrate that highly dispersed Au/TiO2 catalysts show an extraordinary selectivity in the oxidation of propene to the corresponding epoxide (>99%), using a combination of H2 and O2 as oxidant.8–10 H2 was added as a sacrificial reductant which permits the activation of dioxygen at relatively low temperatures (303–393 K) therefore allowing selective oxidation of propene to propene oxide.
Since TS-1 has been shown to be a selective material for the epoxidation of propene with H2O2 as the oxidising species the majority of the early studies used this as a catalyst support. Haruta and co-workers found that Au supported on TS-1 gave high selectivity to propanal above 100 °C however below 100 °C over 90% selectivity to PO could be achieved.11 In addition Moulijn12 and others13 demonstrated that Au/TS-1 catalysts were very stable and could be very selective to the formation of propene oxide. Mechanistic studies showed the important role of the gold nanoparticles in establishing a bidentate propoxy species as an intermediate.14 It is postulated in many mechanistic studies that H2O2 is formed on the Au nanoparticle, rather than a bound peroxy species, which is then capable of either desorbing/adsorbing or migrating on the surface to the tetrahedral Ti site in order to carry out the selective oxidation. In many studies H2O2 itself is not observed or analysed for in the reaction mixtures.
Examples in the patent literature report the direct combination of H2 and O2 in the gas phase at elevated pressures and temperature,15 however, these studies include the presence of acid and halides in the gas phase as stabilisers. In this study we aim to utilise catalysts comprising supported AuPd nanoparticles, which have been extensively shown to have a higher H2O2 synthesis rate than monometallic Au, to investigate if H2O2 can be formed and is able to desorb from the catalyst in the gas phase at atmospheric pressure, without adding stabilisers. Designing catalysts for the synthesis of H2O2 as part of a gas phase process is an important research target. There are numerous potential advantages for the synthesis of H2O2 in the gas phase over the current approaches using combined liquid and gas phase reactants. A convenient gas phase process allows small-scale applications, and this permits simpler process design if the H2O2 is used in a subsequent oxidation reaction. In contrast the liquid phase suffers from the disadvantage of low concentrations of O2 and H2 which limits the rate of reaction; in addition there is the potential for leaching of catalyst components. Additionally, using a gas phase reaction permits more facile fundamental research.
Experimental
Supported bimetallic catalysts were prepared by a previously reported wet impregnation of the appropriate catalyst support with solutions of PdCl2 and HAuCl4. A typical preparation for 1 g of 2.5% Pd/2.5% Au/TiO2 was carried out as follows: PdCl2 (0.0416 g) was added to an aqueous solution of HAuCl4 (2.04 ml, 12.25 g Au/1000 ml) and heated to 80 °C with stirring and left until the PdCl2 had completely dissolved. TiO2 (0.95 g Degussa P25) was then added to the solution and the water allowed to evaporate until the mixture formed a paste. The samples were dried at 110 °C for 16 h and then calcined in static air at 400 °C for 3 h with a ramp rate of 20 °C min−1.Catalytic reactions were carried out in a custom laboratory scale fixed bed reactor with 1/2 inch diameter PTFE tube. The catalyst bed, typically 50 mg unless stated, was suspended vertically in an oven by glass wool above and below. A low catalyst bed length/reactor diameter was used to help prevent back pressure issues. Reaction temperature (40–100 °C) was stabilised before a flow of 2% H2 in synthetic air was introduced (50–200 ml min−1). Reactions were typically carried out for 16 h using dreschel bottles in an ice bath to condense liquid products from the gas phase. After reaction the presence of H2O2 was determined by titrating aliquots of reaction solution with acidified Ce(SO4)2 solution in the presence of ferroin as an indicator. The Ce(SO4)2 solution was standardised against (NH4)2Fe(SO4)2·6H2O using ferroin as an indicator. H2O2 decomposition was measured by comparing the difference in H2O2 concentration before and after the reaction using the same titration method outlined above. Oxidation experiments were carried out by bubbling gases through a solution of 2-propanol (99.5% as purchased) which once passed over the catalyst was condensed in an ice bath. NMR analysis of the reaction mixtures was then carried out to identify the presence of oxidation products.
The TAP experiments were performed using a TAP-2 system. A gas mixture containing 2% H2 in air was pulsed over the catalyst from a reservoir at a pressure of 0.6 bar. The reactor was packed with 0.045 g of powdered catalyst held in place with SiC inert packing. The gas exiting the reactor bed was detected via quadrupole mass spectrometer. The response for mass 34 (H2O2) and mass 2 (H2) was monitored over a time of 2 s for each pulse, giving a curve of intensity against time. The experiment was carried out at 60 °C and 155 °C, with a train of 20 pulses averaged per experiment.
Results
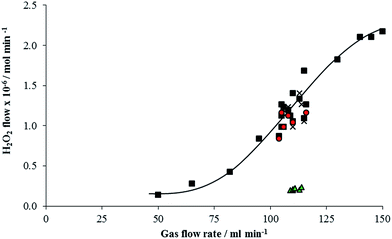
Initial experiments were carried out with the laboratory scale reactor to ensure that any H2O2 produced could pass through the reactor system without significant decomposition occurring. A H2O2 vaporisation calibration curve was established by flowing synthetic air through a 50 wt% H2O2 solution and measuring the moles of H2O2 carried in the vapour phase at various flow rates by condensing the gas phase mixture after the reactor (Fig. 1). The amounts of H2O2 in the gas phase suggest that the gas is not saturated with H2O2, due to the high gas flow rate used and the low vapour pressure of H2O2 at ambient temperature. Following this reactor tubes made of stainless steel and PTFE were trialled, stainless steel decomposed over 90% of the H2O2 vaporised in the gas phase. When a PTFE tube was used the number of moles of H2O2 carried through the tube in the gas phase matched the calibration curve in both synthetic air and 2% H2 in air as shown in Fig. 1. All further experiments were carried out with a PTFE reaction tube.Support materials that had been previously used to support AuPd nanoparticles for H2O2 synthesis were screened for H2O2 decomposition activity in the gas phase reactor (50 wt% H2O2 145 ml min−1 gas flow corresponding to 2.17 mol min−1 from calibration in Fig. 1). Table 1 shows that all supports tested showed significant H2O2 decomposition, which is in contrast to the observed decomposition activity in the liquid phase.16–19
| Support | H2O2 decomposed/% |
|---|---|
| a Reaction conditions: catalyst mass 10 mg, 25 °C, 145 ml min−1 gas flow. | |
| Fe2O3 | 91 |
| Carbon | 85 |
| CeO2 | 77 |
| MgO | 68 |
| TiO2 | 58 |
| SiO2 | 53 |
| Al2O3 | 47 |
This can be attributed to the high surface concentrations of H2O2 passing over the catalyst without the protection of solvation that is available in the liquid phase. Despite these high observed decomposition rates we focused this study on a 2.5% Au 2.5% Pd/TiO2 catalyst to investigate whether oxidative species could be generated from H2 and O2 mixtures on AuPd particles in the gas phase and if H2O2 could be synthesised and detected despite the high decomposition rates of the catalyst support materials.
The oxidation of 2-propanol to acetone was used to probe the presence of oxidative species formed by the catalyst. The gas phase oxidation of 2-propanol using Au catalysts and oxygen has been previously studied by Rossi et al.20 who showed that oxidation occurred at 393 K with over 80% selectivity to carbonyl derivatives. Other studies have shown that Au/TiO2 is also active for the reaction using oxygen with light-off curves beginning at 350–400 K.21 To avoid any reaction with oxygen alone we conducted gas phase oxidation reactions at 60 °C. Gases were bubbled through a solution of 2-propanol (99.5%), lines exiting the catalyst bed were passed through an ice bath so that remaining 2-propanol and liquid products could be condensed for analysis. NMR analysis of the resulting reaction mixtures was then carried out to identify qualitatively the presence of oxidation products.
Initially reactions were carried out with synthetic air and 2% H2 in synthetic air without the presence of a catalyst, in both cases no oxidation products were detected by NMR (Table 2), similar results were observed when using bare TiO2. Also, when the catalyst was present with 2-propanol in synthetic air no oxidation products were detected. However, when 2% H2 in air was used as reactant gas NMR analysis showed the presence of acetone in the reaction solutions condensed after passing over the catalyst. This confirms that, at 60 °C, the AuPd particles are able to activate oxygen in the presence of H2 to oxidise organic molecules such as 2-propanol. This temperature is lower than reported for oxygen alone indicating that H2 can initiate the oxidation at lower temperature, probably through the formation of a surface bound – hydroperoxy intermediate or through the formation of free H2O2 which is further activated to carry out the oxidation.
| Entry | Catalyst | Gas mixture | Oxidation to acetone |
|---|---|---|---|
| a Reaction conditions: catalyst mass 50 mg, 60 °C, 50 ml min−1 gas flow – reaction products qualitatively analysed by NMR. | |||
| 1 | None | Industrial grade air | No |
| 2 | None | 2% H2/air | No |
| 3 | TiO2 | Industrial grade air | No |
| 4 | TiO2 | 2% H2/air | No |
| 5 | 5% AuPd/TiO2 | Industrial grade air | No |
| 6 | 5% AuPd/TiO2 | 2% H2/air | Yes |
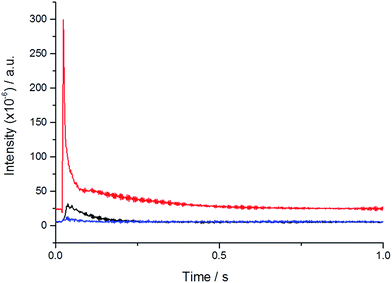
Temporal analysis of products (TAP) reactor studies were carried out to investigate whether H2O2 is formed and able to desorb from the catalyst surface. Reactions were carried out at 60 °C by pulsing 2% H2/air over the catalyst bed. Fig. 2 shows the accumulated signals observed for mass 34 (H2O2) and 2 (H2) during the pulses.
A large H2 signal is detected at the beginning of each pulse along with a smaller signal at mass 34 corresponding to the mass of H2O2. This result shows that it is possible to generate H2O2 in the gas phase and under the vacuum of the TAP reactor it is possible to desorb the H2O2 from the surface of the catalyst and detect it as a reaction product.
Experiments were also conducted in a continuous fixed bed reactor to test under practical conditions for H2O2 synthesis at various temperatures between 40 and 80 °C using 2% H2 in air at 60 °C. When no catalyst was present with air or 2% H2 in air no condensate was seen after the reactor in the cold trap indicating that there was no detectable moisture in the gas feeds. In the presence of 50 mg of catalyst with the synthetic air mixture again no condensate was seen in the cold trap. When 2% H2 in air was used as the reactant gas, liquid was observed in the cold trap indicating that at 60 °C the AuPd nanoparticles were capable of reacting H2 and O2 in the gas phase. On titration it was determined that the solution contained 53 ppm of H2O2. This result demonstrates that when using a 5% AuPd/TiO2 catalyst, that is known to have high H2O2 synthesis rates in the liquid phase at 2 °C, it is possible to produce H2O2 in the gas phase reactor system where the residence time is of the order of seconds at ambient pressure. The result also demonstrates that it is possible for H2O2 to desorb from the catalyst into the gas phase after it is formed.
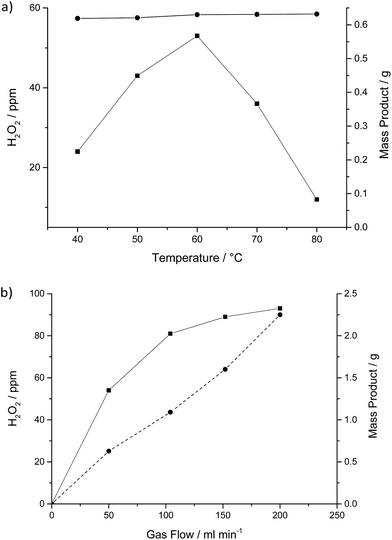
Fig. 3a shows the effect of increasing the reaction temperature from 40–80 °C in the flow reactor. As the temperature is increased the amount of product condensed in the cold trap after 16 h remained constant, full conversion of the H2 in the gas stream was observed across the whole temperature range, explaining why we see no increase in liquid reaction product with increasing temperature.
The amount of H2O2 present in the liquid reaction products formed increased from 23 ppm at 40 °C to 55 ppm at 60 °C before decreasing at temperatures above 60 °C. This indicates that there is a balance between the synthesis/desorption and stability of H2O2 through the catalyst bed. H2O2 synthesis rates over the duration of the experiments were approximately 1 × 10−3 mol kgcat−1 h−1, due to the high H2 conversion and extremely low selectivity leading to mainly H2O production. The observation that it is possible to detect H2O2 at all suggests that the water produced could be the result of a H2O2 decomposition pathway rather than combustion of hydrogen and oxygen without the intermediate production of H2O2. These rates are significantly lower than reported rates in the liquid phase at elevated pressure under optimum conditions (64 mol kgcat−1 h−1).
Fig. 3b shows the effect of varying the total gas flow through the catalyst bed over the 16 h reaction. With increasing gas flow the amount of liquid product observed increased linearly, in agreement with the complete conversion of H2 passing over the catalyst. The concentration of H2O2 in the liquid phase increases over the range studied presumably because the faster gas flows can more effectively strip the H2O2 from the catalyst bed.
As no liquid condensate is seen from the gas phase without the presence of the catalyst we can conclude that the water formed is through the combination of H2 and O2 over the catalyst bed. Despite this, the results show that it is possible to produce and collect H2O2 in the gas phase although at low concentrations. Preventing the subsequent decomposition of H2O2 through the catalyst bed remains a challenge never the less this study shows that the concept of producing H2O2 in the gas phase is can be realised.
Conclusions
We demonstrate for the first time that the H2O2 synthesis rates of catalysts that are well studied in the liquid phase with high pressure gas phase reagents are, in fact, high enough to produce H2O2 in the gas phase at atmospheric pressure. Using TAP analysis we have shown that H2O2 can be synthesised and desorbed from the catalyst surface in the gas phase suggesting that gas phase direct synthesis of H2O2 could be feasible. This observation goes some way to explaining the increased reaction rates observed for propene epoxidation when H2 is added to the reactant gas stream. In this study H2 conversions were high and selectivity towards H2O2 was extremely low due to the nature of the packed bed however detectable amounts of H2O2 were synthesised. Through future reactor design and optimization of conditions, coupled with catalyst improvements a simpler process design might be feasible to produce H2O2; the results enable fundamental investigation of the reaction possible through operando surface sensitive spectroscopies.Acknowledgements
We would like to acknowledge Solvay for financial support of A. A.Notes and references
- J. K. Edwards, S. J. Freakley, R. J. Lewis, J. C. Pritchard and G. J. Hutchings, Catal. Today, 2015, 248, 3–9 CrossRef CAS.
- V. R. Choudhary, C. Samanta and P. Jana, Appl. Catal., A, 2007, 317, 234–243 CrossRef CAS.
- P. Blasi, P. Cann, F. Menegazzo, F. Pinna and T. O. Salmi, Ind. Eng. Chem. Res., 2012, 51, 8883–8890 CrossRef.
- M. Piccinini, J. K. Edwards, J. A. Moulijn and G. J. Hutchings, Catal.: Sci. Technol., 2012, 2, 1908–1913 CAS.
- J. K. Edwards, B. Solsona, E. Ntainjua N., A. F. Carley, A. A. Herzing, C. J. Kiely and G. J. Hutchings, Science, 2009, 323, 1037–1041 CrossRef CAS PubMed.
- A. S. K. Hashmi, R. Döpp, C. Lothschütz, M. Rudolph, D. Riedel and F. Rominger, Adv. Synth. Catal., 2010, 352, 1307–1314 CrossRef CAS.
- A. S. K. Hashmi, C. Lothschütz, R. Döpp, M. Rudolph, T. D. Ramamurthi and F. Rominger, Angew. Chem., Int. Ed., 2009, 48, 8243–8246 CrossRef CAS PubMed.
- T. Hayashi, K. Tanaka and M. Haruta, J. Catal., 1998, 178, 566–575 CrossRef CAS.
- M. Haruta, B. S. Uphade, S. Tsubota and A. Miyamoto, Research Journal Chemistry, 1998, 24, 329–336 CAS.
- B. S. Uphade, Y. Yamada, T. Akita, T. Nakamura and M. Haruta, Appl. Catal., A, 2001, 215, 137–148 CrossRef CAS.
- B. S. Uphade, S. Tsubota, T. Hayashi and M. Haruta, Chem. Lett., 1998, 27, 1277–1278 CrossRef.
- T. A. Nijhuis, B. J. Huizinga, M. Makkee and J. A. Moulijn, Ind. Eng. Chem. Res., 1999, 38, 884–891 CrossRef CAS.
- E. E. Stangland, K. B. Stavens, R. P. Andres and W. N. Delgass, J. Catal., 2000, 191, 332–347 CrossRef CAS.
- T. A. Nijhuis, T. Visser and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2005, 44, 1115–1118 CrossRef CAS PubMed.
- M. Nystrom, J. Wanngard and W. Herrmann, US 006299852B1, 2001.
- J. K. Edwards, A. Thomas, B. E. Solsona, P. Landon, A. F. Carley and G. J. Hutchings, Catal. Today, 2007, 122, 397–402 CrossRef CAS.
- B. E. Solsona, J. K. Edwards, P. Landon, A. F. Carley, A. Herzing, C. J. Kiely and G. J. Hutchings, Chem. Mater., 2006, 18, 2689–2695 CrossRef CAS.
- J. K. Edwards, B. E. Solsona, P. Landon, A. F. Carley, A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2005, 236, 69–79 CrossRef CAS.
- J. K. Edwards, B. Solsona, P. Landon, A. F. Carley, A. Herzing, M. Watanabe, C. J. Kiely and G. J. Hutchings, J. Mater. Chem., 2005, 15, 4595–4600 RSC.
- S. Biella and M. Rossi, Chem. Commun., 2003, 378–379 RSC.
- M. C. Holz, K. Kähler, K. Tölle, A. C. van Veen and M. Muhler, Phys. Status Solidi B, 2013, 250, 1094–1106 CrossRef CAS.
Footnote |
| † The data contained in this paper are archived at http://dx.doi.org/10.17035/d.2016.0009092041. |
| This journal is © The Royal Society of Chemistry 2016 |