Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of multi-armed triphenylamine-based hole transporting materials for high performance perovskite solar cells†
Sungmin
Park‡
ad,
Jin Hyuck
Heo‡
b,
Jae Hoon
Yun‡
af,
Tae Sub
Jung
cd,
Kyungwon
Kwak
cd,
Min Jae
Ko
ag,
Cheol Hong
Cheon
d,
Jin Young
Kim
e,
Sang Hyuk
Im
*b and
Hae Jung
Son
*af
aPhotoelectronic Hybrid Research Center, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea. E-mail: hjson@kist.re.kr
bFunctional Crystallization Center (FCC), Department of Chemical Engineering, Kyung Hee University, Yongin-si 17104, Gyeonggi-do, Republic of Korea. E-mail: imrom@khu.ac.kr
cDepartment of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea
dDepartment of Chemistry, Korea University, Seoul 02792, Republic of Korea
eDepartment of Materials Science and Engineering, Seoul National University, Seoul 02792, Republic of Korea
fDepartment of Nanomaterials Science and Engineering, University of Science and Technology (UST), Daejeon 34113, Republic of Korea
gKU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul 02841, Republic of Korea
First published on 17th May 2016
Abstract
A series of hole-transporting materials (HTMs) based on [2,2]paracyclophane and triphenyl-amine (TPA) was synthesized. We studied the effect of the chemical structure of the HTM on the photovoltaic performance of perovskite solar cells by varying the number of TPA charge transporting components in the HTM. Tetra-TPA, in which four TPAs are incorporated into the [2,2]paracyclophane core, exhibited better hole transport properties than di-TPA and tri-TPA, which contain two and three TPAs, respectively. In particular, incorporation of the TPA group with a multi-armed structure effectively enhanced the conductivity of the HTM layer in the out-of-plane direction in the solar cell device. Due to the improved charge transport and appropriate molecular energy levels of tetra-TPA, the perovskite solar cell based on the tetra-TPA HTM achieved higher Jsc and FF values than the devices based on di-TPA and tri-TPA HTMs, with a high solar cell efficiency (17.9%).
Organometallic halide perovskite solar cells have emerged as the most promising low-cost photovoltaic technology. The perovskites used in solar cells have the chemical formula ABX3, where A, B, and X are ¼CH3NH3 (or NHCHNH3), ¼Pb, and ¼halide (Br, Cl, or I), respectively. This formula has several advantageous properties for the enhancement of photovoltaic effects such as high light absorption, excellent charge carrier diffusion lengths, and a small exciton binding energy.1–5 Consequently, power conversion efficiencies (PCEs) above 20% have been achieved6 that are comparable to those of crystalline Si solar cells.6,7 Perovskite solar cell devices are typically composed of multiple inter-stacked materials, namely transparent electrodes, electron transporting materials, perovskite photoactive materials, hole-transporting materials, and metal electrodes. Hole-transporting materials (HTMs) are important for achieving high solar cell efficiencies; their roles are to transport holes transferred from the perovskite active layer to the metal electrode and to reduce electron–hole recombination by blocking electron transfer.8,9 One commonly used HTM is spiro-OMeTAD (2,2′,7,7′-tetrakis[N,N-di-p-methoxyphenylamine]-9,9′-spirobifluorene), which performs well irrespective of the perovskite solar cell device architecture.7,10–12 However, the multi-step synthesis necessary for the preparation of spiro-OMeTAD limits its practical applications in perovskite solar cells.13,14 Various molecular and polymeric HTMs have been used in perovskite solar cell devices;2,14–18 however, HTM design is hampered by our poor understanding of the relationship between the chemical structures of HTMs and their charge transport properties. It is therefore vital to develop new HTMs and study the relationship between their molecular structures and the photovoltaic properties of solar cell devices based on them. Herein, we present the syntheses of a series of HTMs that incorporate various numbers of a particular transport component, the triphenyl amine group (TPA), and our assessment of the effects of varying the HTM molecular structure on their electrical properties and performances in perovskite solar cell devices.
Tetra-TPA has four TPAs that are incorporated into a [2,2]paracyclophane core, as shown in Fig. 1. [2,2]Paracyclophane has a simple structure that gives HTMs advantageous structural features, such as a cylindrical and rigid structure,19,20 which promote dense packing. Tri-TPA has one less TPA group than tetra-TPA, while di-TPA is a single component of tetra-TPA that is composed of two N,N,N′′,N′′-tetrakis(4-methoxyphenyl)-[1,1′:4′,1′′-terphenyl]-4,4′′-diamine units bridged via two ethyl groups. The photovoltaic performances of perovskite solar cell devices depend on the chemical structures of the HTM. It was found that the solar cell device employing tetra-TPA exhibits a higher PCE (17.9%) than the di-TPA-based device (15.3%).
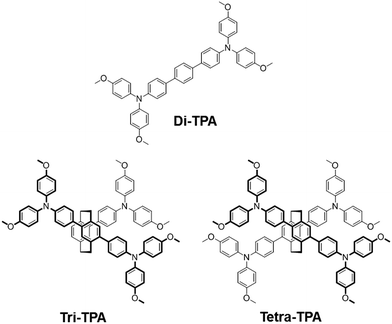 | ||
| Fig. 1 Structures of HTMs containing two, three and four TPAs incorporated into a [2,2]paracyclophane core. | ||
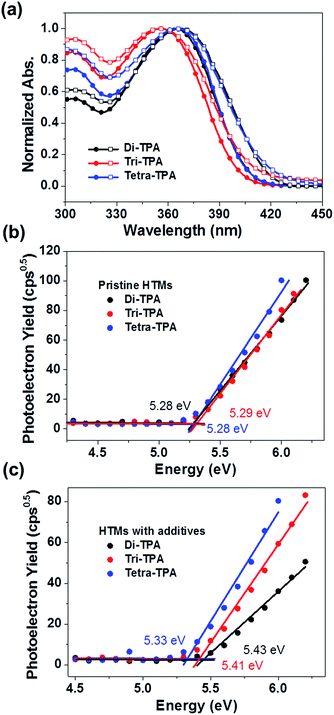
Tetra-TPA and tri-TPA were synthesized by performing the Suzuki cross-coupling reaction of triphenylamine boronic ester with tetra-bromo[2,2]paracyclophane and tri-bromo[2,2]paracyclophane, respectively. The syntheses are described in detail in the ESI.† The UV-vis absorption properties of the HTMs were investigated, as shown in Fig. 2(a) and the characteristic data are summarized in Table 1. The absorption properties of di-TPA and tetra-TPA in solution are very similar, whereas tri-TPA shows a more pronounced first absorption peak at ∼300 nm, with 8–9 nm blue-shifted maximum and onset absorption points compared to those of the other HTMs. The maximum wavelengths of absorption (λmax) for di-TPA, tri-TPA, and tetra-TPA in toluene are 364 nm, 356 nm, and 365 nm, respectively. The λmax absorption values for the HTM films are very similar to the corresponding values for the HTM solutions, although the onset points are red-shifted; interestingly, the red-shifts of di-TPA (13 nm) and tetra-TPA (11 nm) are higher than that of tri-TPA (7 nm), suggesting that the symmetric molecular structures of di-TPA and tetra-TPA are more favorable for intermolecular packing compared to that of asymmetric tri-TPA.21 The energy bandgaps (Eg) of the HTMs were calculated from the wavelengths of the intersections of the absorption and emission spectra of the films.21 The optical bandgaps of di-TPA, tri-TPA, and tetra-TPA are 2.98 eV, 2.95 eV, and 2.96 eV, respectively. Fig. S10† shows the PL spectra of the three HTMs in solution and in the solid state: the Stokes shifts of tri-TPA and tetra-TPA are ∼20 nm larger than that of di-TPA. In general, the Stokes shift depends on the relaxation to the energy-minimized geometry at the excited state after vertical excitation;22,23 hence, di-TPA is likely to undergo a smaller geometric relaxation than the other molecules.
 | ||
| Fig. 2 (a) UV/vis spectra of HTMs in toluene (●) and film (□). (b and c) PESA data of HTMs with and without additives; the lines are linear fits of the data. | ||
| HTMs | λ max (nm) (solution (film)) | λ onset (nm) (solution (film)) | λ em (nm) (film) | E g (eV) |
|---|---|---|---|---|
| a Maximum emission excited at λmax. | ||||
| Di-TPA | 364 (367) | 405 (418) | 453 | 2.98 |
| Tri-TPA | 356 (355) | 400 (407) | 462 | 2.95 |
| Tetra-TPA | 365 (366) | 405 (416) | 465 | 2.96 |
The thermal properties of the HTMs were obtained using differential scanning calorimetry (DSC), as shown in Fig. S11.† The glass transition temperatures (Tg) of the HTMs were determined during the second cycle of heating; the values were found to be 81.6 °C, 119.5 °C, and 148.5 °C for di-TPA, tri-TPA, and tetra-TPA, respectively. The Tg of an organic molecule depends on its rigidity (or flexibility) and can vary due to steric hindrance.24 Di-TPA has a relatively low Tg, likely because the para-terphenyl core has a higher rotational freedom than the tri-TPA and tetra-TPA cores, i.e. [2,2]paracyclophane. Among the three HTMs, tetra-TPA has the highest Tg because of its dense and rigid structure.
The HOMO energy levels of the HTMs were determined from thin films using photoelectron spectroscopy in air (PESA),15,25 and their HOMO energy level changes before and after addition of tert-butyl pyridine (tBP) and lithium bis(trifluoromethylsulfonyl)imide salt (Li-TFSI) additives were compared, as shown in Fig. 2(b and c). Without the additive, all HTMs showed rather similar HOMO values to each other, with −5.28 eV, −5.29 eV, and −5.28 eV for di-TPA, tri-TPA, and tetra-TPA, respectively. However, with the additive, the HOMO energy level of di-TPA was significantly decreased to −5.43 eV, which is a slightly lower value than −5.41 eV of tri-TPA with the additive. Tetra-TPA showed the smallest decrease after adding the additive and thus, the highest HOMO level of −5.33 eV among the HTMs. This is probably because of more effective delocalization of a radical cation over the tetra-TPA molecule, compared with di-TPA and tri-TPA. The reorganization energies (λh) of the HTMs, which represent their relaxation after oxidation, were calculated using the density functional theory (DFT) method with the B3LYP functional and 6-311G(d,p) basis set.26–28 A smaller reorganization energy implies that holes are transferred more efficiently from the perovskite layer to the HTM, as long as the driving force for charge transfer, the HOMO energy offset between the perovskite and HTM layer, is constant. Di-TPA has the lowest calculated reorganization energy (λh = 0.147 eV), followed by tri-TPA (λh = 0.248 eV) and tetra-TPA (λh = 0.595 eV). Therefore, it is expected that the efficiency of hole transfer to the HTM layer decreases in the order di-TPA > tri-TPA > tetra-TPA.
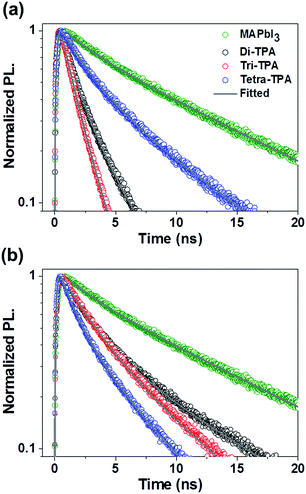
The time-resolved photoluminescence (TR-PL) decays of the HTMs were studied to compare the hole injection into layers of these HTMs from the CH3NH3PbI3 (MAPbI3) absorbing layer. PL decay times (τe) of the prepared films, where τe is the time required for the PL to fall to 1/e of its initial intensity,4 were measured to compare the MAPbI3 PL lifetimes of the films, as shown in Fig. 3. The τe value of the pristine MAPbI3 is 10.06 ns. When the HTMs without additives were stacked on the perovskite layer, the τe values of MAPbI3 were found to be significantly reduced from that of the pristine MAPbI3 film and to be dependent on the HTM. In particular, the τe value of tetra-TPA (4.62 ns) is more than twice those of di-TPA (2.08 ns) and tri-TPA (1.57 ns). Hole transfer from the MAPbI3 layer to the HTM is more efficient for di-TPA and tri-TPA than for tetra-TPA, which could be because the reorganization energies of di-TPA and tri-TPA are lower than that of tetra-TPA due to the lower energy cost of receiving a hole for these molecules. However, when the additives were included in the HTM, a different trend was found in τe: di-TPA and tri-TPA exhibit τe values of 4.54 ns and 4.27 ns, respectively, which are longer than the corresponding values obtained in the absence of the additives. In contrast, the τe value of tetra-TPA is slightly reduced to 3.14 ns after the addition of the additives and is even lower than those of di-TPA and tri-TPA. These results are mainly associated with the HOMO energy levels of the HTMs: the decreases in the HOMO energy levels of di-TPA and tri-TPA are larger compared to that of tetra-TPA after the addition of the additives, which results in smaller energy offsets between the HOMO energy levels of these HTMs and the perovskite layers (valence band edge of MAPbI3 = −5.46 eV),15 and thus in lower driving forces for charge transfer than that of tetra-TPA. Despite their lower reorganization energies, the smaller driving forces for charge transfer of di-TPA and tri-TPA result in less efficient hole transfer in solar cell devices.
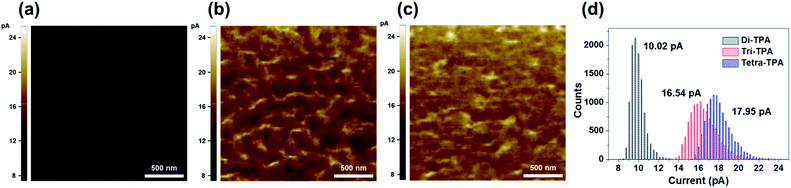
The hole mobility values were measured using the space charge limited current (SCLC) method. Tetra-TPA has the largest hole mobility value, 6.32 × 10−4 cm2 V−1 s−1, followed by tri-TPA, 5.1 × 10−4 cm2 V−1 s−1 and di-TPA, 3.8 × 10−5 cm2 V−1 s−1 (Fig. S12†). X-ray diffraction (XRD) data indicated that all the HTMs have similar amorphous properties in films; hence the differences between the mobilities of the HTMs are not related to their film packing structures. Conductive atomic force microscopy (c-AFM) was performed on the HTM films to obtain their current maps. As shown in Fig. 4, there is a higher electric current over the whole tetra-TPA film than for the other films and the di-TPA film exhibits the lowest current. These results indicate that charge transport in the out-of-plane direction decreases in the order tetra-TPA > tri-TPA > di-TPA. Thus, it is concluded that the introduction of the TPA groups to create a multi-armed structure in tetra-TPA is favorable for intermolecular charge transport in the amorphous film.
Planar MAPbI3 hybrid solar cells with a device structure of FTO/TiO2/MAPbI3/HTM/Au were prepared using the three HTMs. A representative SEM cross-sectional image of these devices is shown in Fig. S13.†Fig. 5 shows the device performances of the planar MAPbI3 hybrid solar cells, and their characteristic photovoltaic properties are summarized in Table 2. The device based on tetra-TPA exhibits the best performance, with a PCE (ηavg) of 17.9% averaged over individual measurements under forward and reverse scan conditions, followed by tri-TPA with a PCE (ηavg) of 16.3%. The solar cell device with di-TPA has the lowest efficiency, PCE (ηavg) = 15.3%. Fig. S14(a–c)† shows histograms of the PCE deviations of 40 devices. The average PCE values were 14.5 ± 2.1%, 13.1 ± 2.0%, and 12.0 ± 1.6% for tetra-TPA, tri-TPA, and di-TPA, respectively. The overall efficiency improvement that results from using tetra-TPA rather than di-TPA arises from the increases in the Jsc and FF values. The external quantum efficiency (EQE) spectra in Fig. 5(d) are consistent with the Jsc results; the tri-TPA and tetra-TPA devices exhibit higher EQE values in the range of 350–750 nm compared with the di-TPA device. Upon illumination, the MAPbI3 layer absorbs light and generates free electrons and holes or loosely bonded electron–hole pairs due to the small exciton binding energy of 30 meV.4 Therefore, most electrons and holes are generated in the perovskite active layer and then transported to the TiO2 electron conductor and HTM, respectively. The improved Jsc and FF values of the tetra-TPA-based solar cell are attributed to the more efficient charge transfer from the perovskite layer to the HTM, as expected from the TR-PL results, and the increased charge transport in tetra-TPA. The improved charge transport properties in the device based on tetra-TPA can be attributed, at least in part, to the multi-armed TPA having a greater capacity for efficient charge transport. We also fabricated solar cell devices with spiro-OMeTAD using the same processing conditions employed for the new HTMs. The PCE is an averaged value from the efficiencies obtained from the forward and reverse scans. The best efficiency is 15.65% and the average value obtained from 40 devices is 12.2 ± 1.9%. Fig. S15(a–c)† shows photovoltaic performance, a EQE spectrum, and a PCE histogram of the device. From the results, it is revealed that di-TPA shows similar performance to that of spiro-OMeTAD. Tri-TPA exhibits improved performance due to its enhanced Jsc. The efficiency of the tetra-TPA based solar cell is 2–3% higher due to improvement in the Jsc and FF values.
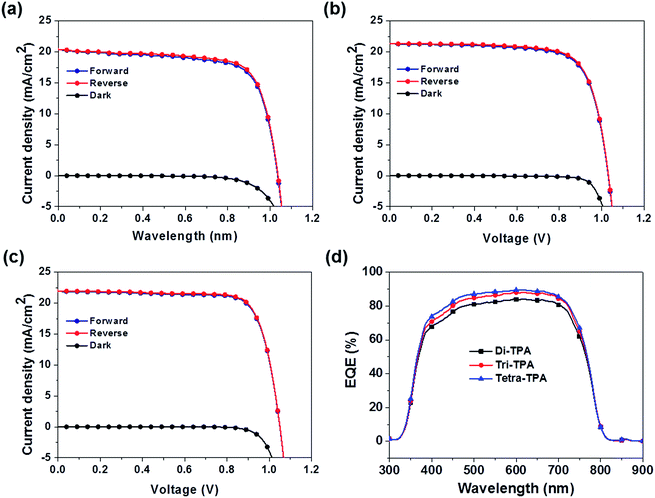 | ||
| Fig. 5 Photovoltaic properties of planar MAPbI3 hybrid solar cells with (a) di-TPA, (b) tri-TPA, and (c) tetra-TPA and (d) EQE spectra. | ||
| HTMs | Scan direction | V oc | J sc | FF (%) | η (%) | η avg (%) |
|---|---|---|---|---|---|---|
| a PCE values averaged over forward and reverse scans. | ||||||
| Di-TPA | Forward | 1.03 | 20.4 | 71.6 | 15.0 | 15.3 |
| Reverse | 1.03 | 20.5 | 73.3 | 15.5 | ||
| Tri-TPA | Forward | 1.03 | 21.4 | 73.6 | 16.2 | 16.3 |
| Reverse | 1.03 | 21.4 | 74.4 | 16.4 | ||
| Tetra-TPA | Forward | 1.05 | 21.8 | 77.7 | 17.8 | 17.9 |
| Reverse | 1.05 | 22.0 | 78.0 | 18.0 | ||
Conclusions
We have developed HTMs with a [2,2]paracyclophane core and investigated the effects of adding TPA units with a multi-armed structure on the photovoltaic properties of perovskite solar cells based on the HTMs. The introduction of the TPA group was found to play an important role in enhancing the charge transport in the amorphous HTM film and thus in improving the perovskite solar cell performance. Due to the efficient charge transfer and transport properties, the perovskite solar cell fabricated with tetra-TPA exhibits higher Jsc and FF values, and thus a higher solar cell efficiency of 17.9%, compared with the corresponding devices prepared with di-TPA and tri-TPA. The present results provide insights for the development of HTMs and thus for the fabrication of efficient perovskite solar cells.Acknowledgements
This work was supported by the Global Frontier R&D Program on Center for Multiscale Energy System and Basic Science Research Program (2015R1A1A1A05001115) funded by the National Research Foundation under the Ministry of Science, Korea Institute of Science and Technology (KIST, 2E26520), and Nano Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant 2012M3A7B4049989). K. K. thanks the NRF for its support (no. 2013R1A1A1010130). The authors thank Dongkyun Seo in Department of Chemistry at Chung-Ang University for assistance with the DFT calculations.References
- J. M. Ball, M. M. Lee, A. Hey and H. J. Snaith, Energy Environ. Sci., 2013, 6, 1739–1743 CAS.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H.-j. Kim, A. Sarkar, M. K. Nazeeruddin, M. Gratzel and S. I. Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS.
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812–2824 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. Sun, S. S. Lim, Y. M. Lam, M. Grätzel, S. Mhaisalkar and T. C. Sum, Science, 2013, 342, 344–347 CrossRef CAS PubMed.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. I. Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- N. J. Jeon, H. G. Lee, Y. C. Kim, J. Seo, J. H. Noh, J. Lee and S. I. Seok, J. Am. Chem. Soc., 2014, 136, 7837–7840 CrossRef CAS PubMed.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963–2967 CAS.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- P. Docampo, J. M. Ball, M. Darwich, G. E. Eperon and H. J. Snaith, Nat. Commun., 2013, 4, 2761–2766 Search PubMed.
- D. Liu and T. L. Kelly, Nat. Photonics, 2014, 8, 133–138 CrossRef CAS.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed., 2014, 53, 4085–4088 CrossRef CAS PubMed.
- P. Gratia, A. Magomedov, T. Malinauskas, M. Daskeviciene, A. Abate, S. Ahmad, M. Grätzel, V. Getautis and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2015, 54, 11409–11413 CrossRef CAS PubMed.
- S. Ryu, J. H. Noh, N. J. Jeon, Y. Chan Kim, W. S. Yang, J. Seo and S. I. Seok, Energy Environ. Sci., 2014, 7, 2614–2618 CAS.
- P. Qin, N. Tetreault, M. I. Dar, P. Gao, K. L. McCall, S. R. Rutter, S. D. Ogier, N. D. Forrest, J. S. Bissett, M. J. Simms, A. J. Page, R. Fisher, M. Grätzel and M. K. Nazeeruddin, Adv. Energy Mater., 2015, 5, 1400980–1400984 Search PubMed.
- L. Cabau, I. Garcia-Benito, A. Molina-Ontoria, N. F. Montcada, N. Martin, A. Vidal-Ferran and E. Palomares, Chem. Commun., 2015, 51, 13980–13982 RSC.
- P. Ganesan, K. Fu, P. Gao, I. Raabe, K. Schenk, R. Scopelliti, J. Luo, L. H. Wong, M. Gratzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 1986–1991 CAS.
- B. König, B. Knieriem and A. D. Meijere, Chem. Ber., 1993, 126, 1643–1650 CrossRef.
- S. Amthor and C. Lambert, J. Phys. Chem. A, 2006, 110, 1177–1189 CrossRef CAS PubMed.
- G. He, Z. Li, X. Wan, J. Zhou, G. Long, S. Zhang, M. Zhang and Y. Chen, J. Mater. Chem. A, 2013, 1, 1801–1809 CAS.
- D. Huang, Y. Chen and J. Zhao, Dyes Pigm., 2012, 95, 732–742 CrossRef CAS.
- Y. Chen, J. Zhao, H. Guo and L. Xie, J. Org. Chem., 2012, 77, 2192–2206 CrossRef CAS PubMed.
- V. Carlier, J. Devaux, R. Legras and P. T. McGrail, Macromolecules, 1992, 25, 6646–6650 CrossRef CAS.
- T. Leijtens, I. K. Ding, T. Giovenzana, J. T. Bloking, M. D. McGehee and A. Sellinger, ACS Nano, 2012, 6, 1455–1462 CrossRef CAS PubMed.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629–6634 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details including synthesis, experimental procedure and supporting data. See DOI: 10.1039/c6sc00876c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |