Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emergence of comparable covalency in isostructural cerium(IV)– and uranium(IV)–carbon multiple bonds†
Matthew
Gregson‡
a,
Erli
Lu‡
a,
Floriana
Tuna
b,
Eric J. L.
McInnes
b,
Christoph
Hennig
cd,
Andreas C.
Scheinost
cd,
Jonathan
McMaster
e,
William
Lewis
e,
Alexander J.
Blake
 e,
Andrew
Kerridge
*f and
Stephen T.
Liddle
*a
e,
Andrew
Kerridge
*f and
Stephen T.
Liddle
*a
aSchool of Chemistry, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: steve.liddle@manchester.ac.uk
bEPSRC National UK EPR Facility, School of Chemistry and Photon Science Institute, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK
cHelmholtz-Zentrum Dresden-Rossendorf, Institute of Resource Ecology, Bautzner Landstrasse 400, D-01314 Dresden, Germany
dThe Rossendorf Beamline, ESRF, BP 220, F-38043 Grenoble, France
eSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
fDepartment of Chemistry, Lancaster University, Lancaster, LA1 4YB, UK. E-mail: a.kerridge@lancaster.ac.uk
First published on 4th February 2016
Abstract
We report comparable levels of covalency in cerium– and uranium–carbon multiple bonds in the iso-structural carbene complexes [M(BIPMTMS)(ODipp)2] [M = Ce (1), U (2), Th (3); BIPMTMS = C(PPh2NSiMe3)2; Dipp = C6H3-2,6-iPr2] whereas for M = Th the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond interaction is much more ionic. On the basis of single crystal X-ray diffraction, NMR, IR, EPR, and XANES spectroscopies, and SQUID magnetometry complexes 1–3 are confirmed formally as bona fide metal(IV) complexes. In order to avoid the deficiencies of orbital-based theoretical analysis approaches we probed the bonding of 1–3via analysis of RASSCF- and CASSCF-derived densities that explicitly treats the orbital energy near-degeneracy and overlap contributions to covalency. For these complexes similar levels of covalency are found for cerium(IV) and uranium(IV), whereas thorium(IV) is found to be more ionic, and this trend is independently found in all computational methods employed. The computationally determined trends in covalency of these systems of Ce ∼ U > Th are also reproduced in experimental exchange reactions of 1–3 with MCl4 salts where 1 and 2 do not exchange with ThCl4, but 3 does exchange with MCl4 (M = Ce, U) and 1 and 2 react with UCl4 and CeCl4, respectively, to establish equilibria. This study therefore provides complementary theoretical and experimental evidence that contrasts to the accepted description that generally lanthanide–ligand bonding in non-zero oxidation state complexes is overwhelmingly ionic but that of uranium is more covalent.
C bond interaction is much more ionic. On the basis of single crystal X-ray diffraction, NMR, IR, EPR, and XANES spectroscopies, and SQUID magnetometry complexes 1–3 are confirmed formally as bona fide metal(IV) complexes. In order to avoid the deficiencies of orbital-based theoretical analysis approaches we probed the bonding of 1–3via analysis of RASSCF- and CASSCF-derived densities that explicitly treats the orbital energy near-degeneracy and overlap contributions to covalency. For these complexes similar levels of covalency are found for cerium(IV) and uranium(IV), whereas thorium(IV) is found to be more ionic, and this trend is independently found in all computational methods employed. The computationally determined trends in covalency of these systems of Ce ∼ U > Th are also reproduced in experimental exchange reactions of 1–3 with MCl4 salts where 1 and 2 do not exchange with ThCl4, but 3 does exchange with MCl4 (M = Ce, U) and 1 and 2 react with UCl4 and CeCl4, respectively, to establish equilibria. This study therefore provides complementary theoretical and experimental evidence that contrasts to the accepted description that generally lanthanide–ligand bonding in non-zero oxidation state complexes is overwhelmingly ionic but that of uranium is more covalent.
Introduction
Ever since the publication of Nature of the Chemical Bond over 75 years ago, chemists have vigorously debated the nature of chemical bonding.1 Nevertheless, it is instructive to conduct research in the chemical sciences within a guiding framework of general bonding descriptions for different areas of the periodic table. Models have suggested variable levels of covalency for transition and early actinide metals whereas the lanthanides and late actinides are generally regarded as being essentially ionic like alkali and alkaline earth metals.2 However, this status quo, especially in f-block chemistry, is continuously being challenged,3 as advances in synthesis and characterisation techniques continuously refine our understanding of these elements.4A comparison of chemical bonding that is often made is between 4f cerium and 5f uranium, since according to Shannon their ionic radii are very similar (0.87 vs. 0.89 Å and 1.01 vs. 1.03 Å for the +IV and +III oxidation states, respectively);5 whilst acknowledging that metal oxidation state and the nature of coordinated ligands directly impact the level of covalency in metal–ligand bonding, for the former the valence 4f-orbitals are generally regarded as ‘core-like’ and chemically inaccessible, whereas for the latter the 5f-orbitals are viewed as chemically accessible and able to engage in modest covalent overlap with ligand frontier orbitals.6 This view finds support from many reactivity and physical measurements, and, for example, the optical spectroscopy and magnetism of uranium complexes is certainly variable and ligand-field-dependent,7 whereas that of lanthanide(III) complexes is generally described as being independent of the ligand environment and ‘free-ion-like’.2,8 However, although scattered throughout the literature there are hints that this appealing yet simple description may be misleading. As an example, for cerium(III) the [Xe]4f1 → [Xe]4f05d1 transition is found to depend strongly on the ligand field, varying from 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737 cm−1 for gaseous Ce3+, to 22
737 cm−1 for gaseous Ce3+, to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 for Ce3+ doped into Y3Al5O12, to 17
000 cm−1 for Ce3+ doped into Y3Al5O12, to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cm−1 for [Ce{η5-C5H3(SiMe3)2}3].9 Furthermore, a number of studies have suggested that the presence of covalent bonding in 4f complexes should be seriously considered.4f,10 An additional point, is that thorium, although exhibiting a larger ionic radius than uranium or cerium (0.94 Å),5 resides like cerium at the start of the f-block and so it is of interest to determine similarities or differences in the chemical bonding of these 4f vs. 5f elements. Overall, for an isostructural pair of tetravalent uranium and cerium complexes, the order of covalency involving those metal centres would normally be expected to be uranium significantly greater than cerium. This is important to understand, from a fundamental perspective, but there are also practical implications; these three elements can be found in the presence of one another in spent nuclear fuel and future strategies to separate them might depend on exploiting differences in their covalent chemical bonding.11 Since f-elements have existing and increasing industrial roles in catalysis, magnets, photonics, alloys, energy, and national security it is increasingly desirable to garner a better understanding of the electronic structure and chemical bonding of these elements.
650 cm−1 for [Ce{η5-C5H3(SiMe3)2}3].9 Furthermore, a number of studies have suggested that the presence of covalent bonding in 4f complexes should be seriously considered.4f,10 An additional point, is that thorium, although exhibiting a larger ionic radius than uranium or cerium (0.94 Å),5 resides like cerium at the start of the f-block and so it is of interest to determine similarities or differences in the chemical bonding of these 4f vs. 5f elements. Overall, for an isostructural pair of tetravalent uranium and cerium complexes, the order of covalency involving those metal centres would normally be expected to be uranium significantly greater than cerium. This is important to understand, from a fundamental perspective, but there are also practical implications; these three elements can be found in the presence of one another in spent nuclear fuel and future strategies to separate them might depend on exploiting differences in their covalent chemical bonding.11 Since f-elements have existing and increasing industrial roles in catalysis, magnets, photonics, alloys, energy, and national security it is increasingly desirable to garner a better understanding of the electronic structure and chemical bonding of these elements.
Despite many studies of uranium, cerium, and thorium complexes, comparative studies of the covalency in their chemical bonding are quite rare, and where documented when this study was initiated usually reinforced standard descriptions,12,13 though there is not a consensus.14 Multi-configurational calculations on uranocene, thorocene, and cerocene return bonding descriptions that order the covalency as uranium > thorium > cerium,12 and studies of M–L (M = U, Ce; L = σ-donor ligand) all suggest the bonding of uranium to be much more covalent than cerium.13 Furthermore, in lanthanide complexes demonstrating some degree of covalent character, calculations have suggested that 5d, 6s and 6p orbitals play a more prominent role in metal–ligand bonding than the 4f.15 At this point, what is to be defined as covalency merits discussion. The mixing coefficient is proportional to the spatial overlap of the orbitals divided by the difference in their energies and the spatial overlap and energy separations are independent parameters.16 Thus, increased covalency may be associated with increased spatial overlap or increased orbital energy near-degeneracy. Although the latter definition is certainly valid, whether it constitutes covalency in the generally chemically accepted view is an interesting question, since covalent chemical bonding carries the connotation of overlap resulting in a build-up of electron density in the inter-nuclear region. It is worth noting at this point that orbital energy levels are not well-defined for all quantum-chemical methodologies, and so probing covalency with an orbital-based computational methodology may not be appropriate. Therefore, this study focuses on an electron density approach rather than orbital structure. This is appropriate in the context of covalency described by spatial overlap; indeed, Pauling referred to covalent bonds as “the sharing of a pair of electrons by the two bonded atoms”.1 This approach permits us to probe exactly this electron sharing in an orthogonal and complementary manner to methods such as XANES ligand K-edge spectroscopy that probe transitions to unoccupied orbitals and extrapolates from this covalency defined on the basis of orbital energy near-degeneracy.4
Recently, as part of a wider effort to prepare lanthanide–carbon multiple bonds,17 we reported the well-defined cerium(IV) carbene diaryloxide complex [Ce(BIPMTMS)(ODipp)2] [1, BIPMTMS = C(PPh2NSiMe3)2; Dipp = C6H3-2,6-iPr2].18 Complex 1 is notable for being a cerium(IV) organometallic and containing a Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C multiple bonding interaction. Whilst dominated by electrostatics, this bond exhibits covalency according to NBO analysis of DFT-derived densities. There is reason to have confidence in such analysis as SAOP/ZORA/TZP TD-DFT calculations at the same level of theory reproduce very well the experimentally observed UV/Vis/NIR spectrum. NBO analysis identifies ∼13% cerium character in each of two Ce–C bonding interactions (σ + π). Non-aqueous cerium(IV)19 is often a difficult oxidation state to access in an organometallic arena,13c,18,20 and the 4th ionisation energy of cerium is greater than the sum of the first three;21 however, with 1 in-hand, we surmised that as uranium(IV) and thorium(IV) are robust oxidation states, the synthesis of 1 presents an opportunity to directly compare the nature of the chemical bonding of cerium, uranium, and thorium. Here, we report the synthesis and characterisation of [M(BIPMTMS)(ODipp)2] (M = U, 2; Th, 3); the synthesis of 2 and 3 are straightforward, but importantly permit a comparison of the bonding of three isostructural complexes. Surprisingly, both DFT (via both orbital- and density-based analyses) and CASSCF/RASSCF (via density-based analysis) methods suggest that the covalency and f-orbital interactions for the cerium and uranium complexes are essentially the same, in contrast to the thorium complex that is essentially ionic. The emergence of these results is in contrast to almost all other examples of comparative studies of 4f and 5f covalency,12,13 and suggests that the established purely ionic general bonding picture of lanthanide cations does not always hold true. Interestingly, this has also recently been suggested by an orthogonal XANES spectroscopy study reported during this work that probed simple cerium(IV) and uranium(IV) hexachloride dianion salts, where on the basis of orbital energy near-degeneracy similar levels of covalency between cerium(IV) and uranium(IV) have been proposed.4f The theoretical description of the relative levels of covalency in 1–3 are also consistent with experimental exchange reactions with metal tetrahalide salts of cerium, uranium, and thorium, further supporting our findings.
C multiple bonding interaction. Whilst dominated by electrostatics, this bond exhibits covalency according to NBO analysis of DFT-derived densities. There is reason to have confidence in such analysis as SAOP/ZORA/TZP TD-DFT calculations at the same level of theory reproduce very well the experimentally observed UV/Vis/NIR spectrum. NBO analysis identifies ∼13% cerium character in each of two Ce–C bonding interactions (σ + π). Non-aqueous cerium(IV)19 is often a difficult oxidation state to access in an organometallic arena,13c,18,20 and the 4th ionisation energy of cerium is greater than the sum of the first three;21 however, with 1 in-hand, we surmised that as uranium(IV) and thorium(IV) are robust oxidation states, the synthesis of 1 presents an opportunity to directly compare the nature of the chemical bonding of cerium, uranium, and thorium. Here, we report the synthesis and characterisation of [M(BIPMTMS)(ODipp)2] (M = U, 2; Th, 3); the synthesis of 2 and 3 are straightforward, but importantly permit a comparison of the bonding of three isostructural complexes. Surprisingly, both DFT (via both orbital- and density-based analyses) and CASSCF/RASSCF (via density-based analysis) methods suggest that the covalency and f-orbital interactions for the cerium and uranium complexes are essentially the same, in contrast to the thorium complex that is essentially ionic. The emergence of these results is in contrast to almost all other examples of comparative studies of 4f and 5f covalency,12,13 and suggests that the established purely ionic general bonding picture of lanthanide cations does not always hold true. Interestingly, this has also recently been suggested by an orthogonal XANES spectroscopy study reported during this work that probed simple cerium(IV) and uranium(IV) hexachloride dianion salts, where on the basis of orbital energy near-degeneracy similar levels of covalency between cerium(IV) and uranium(IV) have been proposed.4f The theoretical description of the relative levels of covalency in 1–3 are also consistent with experimental exchange reactions with metal tetrahalide salts of cerium, uranium, and thorium, further supporting our findings.
Results and discussion
Synthesis and characterisation
In contrast to 1, which required a multi-step preparation,18 the synthesis of 2 and 3 was straightforwardly accomplished by installation of the BIPMTMS carbene then the two aryloxides onto uranium or thorium by sequential salt elimination reactions, Scheme 1. After work-up, complexes 2 and 3 were isolated as brown and colourless crystals in 56 and 61% yield, respectively. The 1H NMR spectrum of 2 spans the range −19 to +17 ppm and the 31P NMR spectrum exhibits a broad resonance at −293 ppm, consistent with the uranium(IV) formulation that is supported by a solution magnetic moment of 2.75 μB at 298 K. In contrast, the 1H NMR spectrum of 3 spans the range 0 to +8 ppm and the 31P NMR resonance appears at +4.7 ppm. The electronic absorption spectrum of 2 (see ESI†) is characterised by weak (ε < 80 M−1 cm−1) absorptions over the range 500–1900 nm that are characteristic of Laporte forbidden f–f transitions for the 3H4 electronic manifold of the 5f2 uranium ion22 whereas for 3 the spectrum is featureless over 400–2000 nm as expected for its colourless 6d05f0 nature. As reported previously, the electronic absorption spectrum of 1 exhibits two broad absorptions in the visible region (435 and 541 nm; ε = 4560 and 5365 M−1 cm−1, respectively), the broadness and resulting purple colour of which is a defining feature of many cerium(IV) complexes.23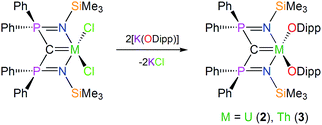 | ||
| Scheme 1 Generic, representative syntheses of 2 and 3. For the synthesis of 1 see ref. 18. | ||
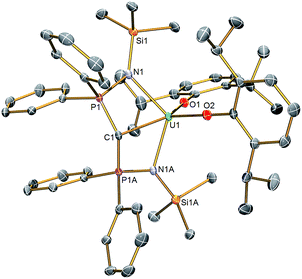
Solid state structures
The molecular structures of 2 and 3 were determined by single crystal X-ray crystallography, Fig. 1 (see ESI†). The salient feature of isostructural 1–3 is a monomeric formulation with terminal M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The remaining coordination sphere of each metal is completed by two BIPMTMS imino chelate arms and the two aryloxide oxygen centres which enforce a pseudo square-based pyramidal geometry. We found a Ce
C bonds. The remaining coordination sphere of each metal is completed by two BIPMTMS imino chelate arms and the two aryloxide oxygen centres which enforce a pseudo square-based pyramidal geometry. We found a Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 2.441(5) Å in 1;18 this is longer than the Ce
C distance of 2.441(5) Å in 1;18 this is longer than the Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds reported in the theoretical models of CeCH2+ and Cp2CeCH2 complexes,15a,24 but CeCH2+ and Cp2CeCH2 are experimentally unknown, sterically unimpeded and, in the case of the former, benefit from the reduced electronic repulsion associated with a net positive charge. For experimentally realised compounds, the Ce
C bonds reported in the theoretical models of CeCH2+ and Cp2CeCH2 complexes,15a,24 but CeCH2+ and Cp2CeCH2 are experimentally unknown, sterically unimpeded and, in the case of the former, benefit from the reduced electronic repulsion associated with a net positive charge. For experimentally realised compounds, the Ce![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance of 1 is amongst the shortest ever reported, except for the special case of fullerene encapsulated Ce2.25 The U
C distance of 1 is amongst the shortest ever reported, except for the special case of fullerene encapsulated Ce2.25 The U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Th
C and Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distances in 2 and 3 were determined to be 2.414(3) and 2.508(5) Å, respectively; on the basis of Shannon's ionic radii5 the former is ∼0.05 Å shorter than would be anticipated but the latter is as would be expected and both are consistent with U
C distances in 2 and 3 were determined to be 2.414(3) and 2.508(5) Å, respectively; on the basis of Shannon's ionic radii5 the former is ∼0.05 Å shorter than would be anticipated but the latter is as would be expected and both are consistent with U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Th
C and Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in BIPMTMS complexes.26
C bonds in BIPMTMS complexes.26
Magnetism and electron paramagnetic resonance spectroscopy studies
The oxidation state assignments of 1–3 are also consistent with solid state magnetic measurements, Fig. 2 (and see ESI†). Complex 2 has a room temperature χT of 0.93 cm3 K mol−1 (equivalent to a magnetic moment 2.73 μB, in agreement with solution studies; χ is the molar magnetic susceptibility) and decreases rapidly on cooling tending to zero, which is typical for 5f2 uranium(IV) that is a magnetic singlet at low temperature.6,27 Complex 3 is diamagnetic, consistent with closed-shell thorium(IV). Studies of 1 give a very small magnetic moment (0.02–0.1 cm3 K mol−1 depending on temperature and diamagnetic corrections) that varies from batch to batch (see ESI†). Magnetic data for the cerium and uranium samples 1 and 2 were corrected for diamagnetic contributions by subtraction of data of the Th complex 2. However, this procedure is not exact, for example not perfectly accounting for the sealed pyrex sample tubes (which have small magnetic impurities) nor the difference between the 4f (Ce) and 5f (Th) ion diamagnetism. Because measured samples of 1 have a very weak paramagnetism, the precise nature of the χT(T) plot (the absolute value, particularly at high temperature, and the shallow slope in Fig. 2) is very sensitive to the correction applied and should not be over-interpreted. There is no such problem for the strongly paramagnetic 2.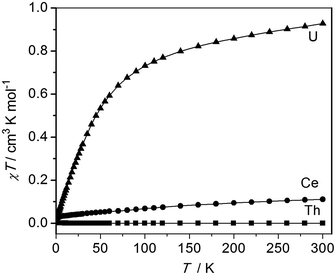 | ||
| Fig. 2 Magnetic data of solid samples of 1–3, measured in 5000 G applied magnetic field. Diamagnetic corrections for 1 and 2 were estimated from the data for 3; the weak paramagnetism from samples of 1 is due to an impurity (see ESI†). | ||
The weak paramagnetic response from samples of 1 is only consistent with a small quantity of paramagnetic impurity where the bulk of the sample is diamagnetic, consistent with cerium(IV) or other diamagnetic configurations (admixtures of cerium(IV) with singlet cerium(III) + radical ligand configurations have been proposed for cerocene).20f The measured paramagnetism is only ca. 5–15% of values expected for f1 cerium(III) (0.8 cm3 K mol−1 calculated for 2F5/2; measured values for anionic cerocene derivatives are 0.6–0.7 cm3 K mol−1 at room temperature decreasing to 0.4–0.6 cm3 K mol−1 at 2 K).20f,28 That the highly air-sensitive 1 decomposes to give cerium(III) – we note the related complex [Ce(BIPMTMS)(ODipp)(THF)]18 is very unstable and decomposes surprisingly easily – is also consistent with low-temperature X-band EPR spectra which have (batch-dependent) broad features at geff,∥ = 3.7 and geff,⊥ = 0.85 (see ESI†). That this is an impurity signal is confirmed by the fact that these geff values would give a magnetic moment of ca. 0.5 cm3 K mol−1 if they derived from the bulk species. Both 2 and 3 are EPR silent, as expected. Although the magnetic and EPR data for 1 are consistent with only the presence of magnetic impurities, they do not, because of the diamagnetic correction being large compared to the weak paramagnetism, rule out excited state mixing, i.e. a multi-configurational ground state, due to the shallow positive gradient that could be attributed to temperature independent paramagnetism. In order to conclusively show that 1 is cerium(IV), and therefore that comparisons to 2 and 3 are valid, we recorded the XANES spectrum of 1.
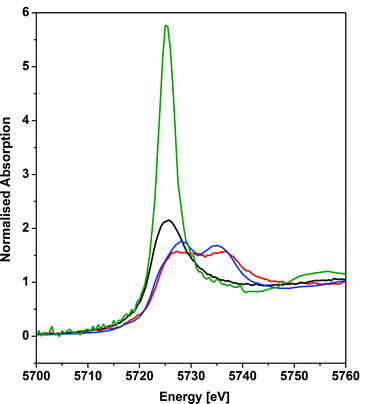
X-ray absorption near edge structure spectroscopy
The cerium LIII-edge spectrum of 0.01 M Ce(NO3)3 in water, the cerium(III) precursor to 1 [Ce(BIPMTMS)(ODipp)2K(THF)], CeO2, and 1 are illustrated in Fig. 3. As expected for cerium(III) complexes, the LIII-edge spectra of aqueous Ce(NO3)3 and [Ce(BIPMTMS)(ODipp)2K(THF)] both consist of a single peak, just above the absorption threshold, at ∼5725.7 eV that is characteristic of cerium(III).29 In contrast, the LIII-edge spectra of CeO2 and 1 both exhibit the characteristic double absorption features of cerium(IV) at ∼5727.2 and ∼5736.7 eV, that are similar to those of CeF4, Ce(SO4)2·4H2O, and CeCl62−.4f,30 The LIII-edge E1 absorption for 1 is ∼1.5 eV higher in energy than the corresponding [Ce(BIPMTMS)(ODipp)2K(THF)] LIII-edge absorption, as found generally for cerium(IV) complexes.4f,29 The double-peak ratio for CeO2 and 1 are both essentially 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as has been found for CeCl62− and CeF4,4f,30 but it is markedly different to that of cerocene.31,32 The double absorptions could be interpreted in different ways, either as a 4f1 L−1 contribution to a multiconfigurational ground state,33 or resulting from final state effects, i.e. a multiconfigurational excited state.4f,34 In this regard, opinion in the literature is divided and the topic is intensively debated, but it is interesting to note that systems with relatively innocent ligands such as chloride and oxide give spectra with features that are energetically similar to more electronically complex molecules such as cerocene, but with different double-peak ratios. Variable-pressure and theoretical studies have both suggested that the double-absorption spectrum of cerium(IV) complexes with oxide and halide ligands is due to final state effects,4f,30d whereas for cerocene this has been attributed to multiconfigurational ground state effects.31 In that regard, the XANES spectrum of complex 1 is certainly much more like that of CeO2, CeF4, Ce(SO4)2·4H2O, and CeCl62− than cerocene; this observation is consistent with the premise that an open-shell singlet or triplet formulation of 1 should be regarded as less likely than a closed-shell singlet and so we conclude that the presence of cerium(III) character in 1 can be excluded.
1, as has been found for CeCl62− and CeF4,4f,30 but it is markedly different to that of cerocene.31,32 The double absorptions could be interpreted in different ways, either as a 4f1 L−1 contribution to a multiconfigurational ground state,33 or resulting from final state effects, i.e. a multiconfigurational excited state.4f,34 In this regard, opinion in the literature is divided and the topic is intensively debated, but it is interesting to note that systems with relatively innocent ligands such as chloride and oxide give spectra with features that are energetically similar to more electronically complex molecules such as cerocene, but with different double-peak ratios. Variable-pressure and theoretical studies have both suggested that the double-absorption spectrum of cerium(IV) complexes with oxide and halide ligands is due to final state effects,4f,30d whereas for cerocene this has been attributed to multiconfigurational ground state effects.31 In that regard, the XANES spectrum of complex 1 is certainly much more like that of CeO2, CeF4, Ce(SO4)2·4H2O, and CeCl62− than cerocene; this observation is consistent with the premise that an open-shell singlet or triplet formulation of 1 should be regarded as less likely than a closed-shell singlet and so we conclude that the presence of cerium(III) character in 1 can be excluded.
Theoretical characterisation
Since the XANES data suggest that 1 possesses formal cerium(IV) character, it can legitimately be compared to 2 and 3. We previously reported Natural Bond Orbital (NBO) data for 1 which returned σ- and π-bonds composed of ∼13% Ce and ∼87% C character.18 In the σ-bond cerium principally employs 4f (76%) and 5d (21%) character whereas the π-bond exhibits high 4f (80%) and 5d (19%) contributions. When interrogating 2 by NBO, a similar breakdown is returned. Specifically, the σ-bond is composed of 16% U and 84% C and the π-bond is made up of 14% U and 86% C character. The U contributions to the σ- and π-bonds are 5f (87%) and 6d (12%), and 5f (77%) and 6d (22%), respectively. In contrast, the NBO data for 3 return ionic interactions with localised carbene lone pairs with no Th character as the contribution of the latter falls below the default cut-off of 5% in the NBO code. These calculations suggest that, whilst the bonding between cerium–, uranium–, and thorium–carbon centres in 1–3 are dominated by ionic interactions, a modest and surprisingly comparable covalent contribution to the bonding is evident in 1 and 2 despite the commonly held view that lanthanide–ligand chemical bonding is purely ionic.Although the NBO calculations are internally consistent and well suited to describing covalency in molecules,35 they are based on results from DFT calculations that have well-documented shortcomings with respect to the treatment of electron correlation. Thus, we turned to multi-configurational calculations to develop a quantitative, meaningful description of the chemical bonding of the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units in 1–3. These calculations employed the restricted-active-space self-consistent field (RASSCF) theory,36 which completely avoids the problems inherent to DFT studies of open-shell systems by treating static electron correlation explicitly via a configuration interaction approach. Whilst RASSCF is a powerful technique for elucidating the nature of metal–ligand interactions in complexes such as those considered here, it is limited in the size of systems to which it can be applied. For this reason, complexes 1–3 were truncated in order to render RASSCF calculations computationally tractable by replacing P-phenyls with H, silyl-methyls with H, and the bulky Dipp groups by Me. This truncation retains the coordination environment of all atoms directly bonded to the metal: where hydrogen termination was employed, only the positions of the terminating hydrogens were optimised.
C units in 1–3. These calculations employed the restricted-active-space self-consistent field (RASSCF) theory,36 which completely avoids the problems inherent to DFT studies of open-shell systems by treating static electron correlation explicitly via a configuration interaction approach. Whilst RASSCF is a powerful technique for elucidating the nature of metal–ligand interactions in complexes such as those considered here, it is limited in the size of systems to which it can be applied. For this reason, complexes 1–3 were truncated in order to render RASSCF calculations computationally tractable by replacing P-phenyls with H, silyl-methyls with H, and the bulky Dipp groups by Me. This truncation retains the coordination environment of all atoms directly bonded to the metal: where hydrogen termination was employed, only the positions of the terminating hydrogens were optimised.
In order to assess any truncation effects on the electronic structures, ground state electron densities were calculated at the PBE/TZVP level of theory. These densities were probed with the quantum theory of atoms in molecules (QTAIM) approach37 since, in contrast to orbital-based measures, multi-configurational studies of cerium(IV) complexes have shown density-based analysis methods provide unambiguous electronic structure interpretations.12,18,38 Furthermore, this density-based approach allows us to consider all contributions to covalent bonding character, irrespective of the orbital origin. We focus on two key properties: the delocalisation index (δ), a quantitative measure of the degree of electron sharing between two atomic centres,39 and the magnitude of the electron density at the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond critical point (ρ), an accepted measure of covalency. These two measures, while complementary, are not equivalent: ρ provides a quantitative measure of charge accumulation in the bonding region, which is related to spatial overlap, whereas the delocalisation index, δ, between two bonded atoms is maximised when electrons are shared equally which, in a monodeterminantal framework, is a manifestation of orbital degeneracy. This analysis therefore allows us to determine the variation in both of these phenomena when the metal centre is varied and has previously been reported in several studies of cerium and uranium complexes.12,13,38,40 Reassuringly, reductions of <2% in ρ and <3% in δ are observed when comparing full and truncated complexes, demonstrating that the quantitative bonding characteristics of the full complexes 1–3 is retained.
C bond critical point (ρ), an accepted measure of covalency. These two measures, while complementary, are not equivalent: ρ provides a quantitative measure of charge accumulation in the bonding region, which is related to spatial overlap, whereas the delocalisation index, δ, between two bonded atoms is maximised when electrons are shared equally which, in a monodeterminantal framework, is a manifestation of orbital degeneracy. This analysis therefore allows us to determine the variation in both of these phenomena when the metal centre is varied and has previously been reported in several studies of cerium and uranium complexes.12,13,38,40 Reassuringly, reductions of <2% in ρ and <3% in δ are observed when comparing full and truncated complexes, demonstrating that the quantitative bonding characteristics of the full complexes 1–3 is retained.
The electronic structures of the truncated complexes were then evaluated using the RASSCF methodology. These calculations employed three active spaces: RAS1, containing only occupied orbitals from the monodeterminantal reference wavefunction, RAS2, containing both occupied and virtual orbitals, and RAS3, containing only virtual orbitals. Full configuration interaction (CI) was performed in RAS2, while truncated CI, considering only singly and doubly excited configurations, was performed between the RAS1, RAS2 and RAS3 subspaces. All active space orbitals were optimised. Due to the large computational costs of such calculations, the RAS1, RAS2 and RAS3 subspaces were restricted to 11, 7 and 11 orbitals, respectively: the 7 RAS2 orbitals incorporated the 4f/5f manifold, whereas the RAS1 and RAS3 subspaces included all orbitals with significant carbon and nitrogen 2s and 2p character. The oxygen 2s and 2p orbitals could not be included in the active subspaces, since attempts resulted in the intrusion of phosphorus-based orbitals. It was observed, however, that oxygen 2s and 2p orbitals that were successfully stabilised in the active subspaces exhibited occupation numbers extremely close to integer values. Similarly, occupation numbers of formally unoccupied d-orbitals was effectively 0, indicating that the inclusion of these orbitals in the active subspaces is not required. It may be that the geometric constraints of BIPMTMS favour f- over d-orbital participation in the bonding of this ligand to f-elements generally, but further studies will be required to confirm this. This definition of the active subspaces resulted in RASSCF(n,2,2;11,7,11) calculations. The number of explicitly correlated electrons, n, was 22 for complexes 1 and 3 and 24 for complex 2. In all cases, calculations were performed in Cs symmetry.
The results of these calculations reveal that all complexes are dominated by electronic configurations corresponding to metal(IV) centres, in agreement with our experimental measurements, and these configurations contribute 89.0, 89.5 and 89.3% to the ground state RASSCF wavefunctions of 1–3 (which are of 1A′, 3A′′, and 1A′ symmetry), respectively. Maximum deviations from integer values in natural orbital occupations were 0.032, 0.033, and 0.025, respectively, indicating rather weak multi-configurational character.41 The lack of strong multi-configurational character in the cerium complex, supported by experimental data, is in stark contrast to that found in cerocene.12a,20d,20f,38a,42 For all complexes under consideration, it was found that only the natural orbitals of σ and π M–C (anti-)bonding character exhibited significant deviation from integer occupation, indicating that a simplified complete active space (CAS) comprising 4 electrons correlated in 4 orbitals (or 6 electrons in 6 orbitals to incorporate the 5f2 configuration of the uranium compound) should be sufficient to accurately describe the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonding interaction. Subsequent analysis of CASSCF-derived densities revealed them to be extremely similar to their RASSCF counterparts (see Tables S15 and S16 of the ESI†). In the following discussion, however, all quantities are derived from RASSCF calculations.
C bonding interaction. Subsequent analysis of CASSCF-derived densities revealed them to be extremely similar to their RASSCF counterparts (see Tables S15 and S16 of the ESI†). In the following discussion, however, all quantities are derived from RASSCF calculations.
In Fig. 4 we present relevant natural orbitals for each complex. The similarity of these orbitals in the cerium and uranium complexes, as well as the near-identical occupation numbers, is startling. In both cases clear σ- and π-bonding character can be seen, in contrast to the ligand-localised orbitals in the thorium complex. The two singly-occupied 5f orbitals in the uranium complex are almost entirely localised on the uranium centre (∼98% 5f character), with negligible ligand contributions.
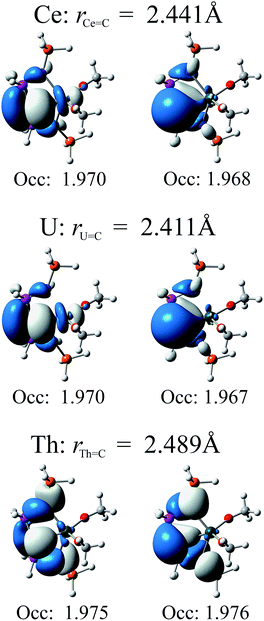 | ||
| Fig. 4 Relevant natural orbitals and corresponding occupancies obtained from RASSCF calculations on the truncated complexes. All orbitals rendered using an isosurface value of 0.04. | ||
The RASSCF-calculated wavefunctions were used to obtain explicitly correlated electron densities for subsequent QTAIM analysis. Metal charges are all significantly higher than those found using DFT, increasing by 0.68, 0.59 and 0.49 to give absolute values of +2.84, +2.89 and +3.02 a.u. for 1–3, respectively, and result in very similar cerium and uranium charges which are notably lower than the thorium charge, indicating greater ionic character in the latter. An increase in ρ is also found, but is less pronounced: 0.0051, 0.0020 and 0.0028 a.u., to give absolute values of 0.0784, 0.0859 and 0.0756 a.u., respectively. The increase is most pronounced in the cerium complex, and results in covalent character larger than that found in the thorium analogue. Whilst an overall reduction in δ is found, −0.238, −0.229, −0.120 a.u. to give absolute values of 0.538, 0.543 and 0.493 a.u. for 1–3, respectively, the resulting values also indicate higher covalency in the cerium complex when compared to the thorium analogue and, indeed, demonstrate covalent character of almost the same magnitude as that found in the uranium complex. When combined, these data provide strong evidence for ordering the extent of covalency in these complexes as uranium ≈ cerium > thorium in these complexes and thus confirm the premise suggested by the DFT calculations, in stark contrast to previous studies. For example, in the cerium(IV) N-heterocyclic carbene complex [Ce(L){N(SiMe3)2}2F] [L = OCMe2CH2(CNCH2CH2N-Dipp)] a ρ(Ce,C) of 0.045 a.u. is found,13c which is 81.8% of the value found in the analogous uranium complex. Chloro analogues were also considered and ρ(Ce,C) was found to be 84.9% of the ρ(U,C) value. Similarly, calculations on cerocene11 found ρ(Ce,C) to be 0.0395, which is 83.0% of the analogous value calculated for uranocene.12a Here, we find ρ(Ce,C) for 1 to be 91.3% of the corresponding U value in 2. Comparison of δ(Ce,C) can also be made with that in cerocene, where it was found to be 83.2% of the δ(U,C) value in uranocene; here, we calculate δ(Ce,C) for 1 to be 99.1% of the corresponding δ(U,C) value in 2.
Exchange reactions and thermodynamic considerations
In order to experimentally probe the relative levels of covalency in 1–3 and calibrate the above calculations, we investigated the exchange reaction chemistry of 1–3 since it is well known that covalency can drive exchange reactions. For example, rare earth tris-cyclopentadienyl complexes readily react with iron halides to afford ferrocene and rare earth halides and this reactivity, whilst undoubtedly reflecting the favourable formation of lanthanide–halide bonds, is driven by the formation of highly covalent iron–cyclopentadienyl bonding.43| (1) + [ThCl4(THF)3.5] → no reaction | (1) |
| (3) + [CeCl4(HMPA)2] → reaction | (2) |
Apart from the onset of decomposition of 1, which is known to be unstable in solution, no reaction between 1 and [ThCl4(THF)3.5] is observed in benzene after a 24 hour stir (eqn (1)). After a 5 day stir 1 is completely decomposed to yield a species that exhibits a resonance at −34 ppm in the 31P NMR spectrum. Although we have not been able to isolate and identify this species its 31P NMR chemical shift is in the region where related cerium(III) BIPMTMS complexes exhibit 31P NMR resonances.18 It would therefore seem that 1 does not react with thorium tetrachloride and instead decomposes before any reactivity can occur. In the reverse scenario, eqn (2), treatment of 3 with [CeCl4(HMPA)] [HMPA = OP(NMe2)3] results in the loss of 1H NMR resonances attributable to 3 and evolution of the characteristic purple colour of 1 in the first 15 minutes. After 15 minutes the purple colour fades and an intractable mixture of products is formed. Given that the preparation of 1 is not straightforward it is not surprising that if formed under these less than optimal conditions it would decompose given its instability in solution, but the purple colour is certainly consistent with the exchange of BIPMTMS from thorium(IV) to cerium(IV) and in-line with the proposed differences in covalency.
| (2) + [ThCl4(THF)3.5] → no reaction | (3) |
| (3) + [UCl4(THF)3] → reaction | (4) |
As with the absence of reaction between 1 and [ThCl4(THF)3.5], eqn (1), we find that there is also no reaction of 2 with [ThCl4(THF)3.5], eqn (3). In the reverse situation, eqn (4), 3 does react with [UCl4(THF)3]. Unfortunately, an intractable product mixture is obtained, likely due to ligand scrambling under conditions that are by definition less controlled than the usual route to prepare 2. However, it is clear that 1H NMR resonances attributable to 3 are lost so the implication is that the BIPMTMS ligand is transferred to uranium. Irrespective of the precise outcomes, these reactions are consistent with uranium being more covalent than thorium.
| (1) + [UCl4(THF)3] → reaction | (5) |
| (2) + [CeCl4(HMPA)2] → reaction | (6) |
When 1 is treated with [UCl4(THF)3], eqn (5), the intense purple colour of 1 fades within 30 minutes and is replaced by a green colour which is then replaced by a brown colour consistent with the formation of 2. In the reverse situation, eqn (6), 1H NMR resonances attributable to 2 are lost; no purple colour was observed, but it is clear that ligand exchange has occurred, and we note that after a 5 day stir the mixture exhibits a 31P NMR resonance at −34 ppm, which is indicative of a cerium(III) BIPMTMS derivative.18 In order to exclude the possibility that decomposition is due to HMPA we treated 2 with neat HMPA in a control experiment and found that no reaction occurs. The uranium–cerium exchange reactions are not clean, but it is evident that ligand exchange occurs to some extent. Although equilibria are to some extent established, 1 is not stable in solution for extended periods and the evidence suggests that eventually the cerium decomposes to the trivalent state, which then degrades the equilibria.
| (1) + [ThCl4(THF)3] → (3) + [CeCl4(THF)3], ΔHrxn = +11.2 kcal mol−1 | (7) |
| (3) + [CeCl4(HMPA)2] → (1) + [ThCl4(HMPA)2], ΔHrxn = −10.2 kcal mol−1 | (8) |
| (2) + [ThCl4(THF)3] → (3) + [UCl4(THF)3], ΔHrxn = +13.5 kcal mol−1 | (9) |
| (3) + [UCl4(THF)3] → (2) + [ThCl4(THF)3], ΔHrxn = −13.5 kcal mol−1 | (10) |
| (1) + [UCl4(THF)3] → (2) + [CeCl4(THF)3], ΔHrxn = −2.3 kcal mol−1 | (11) |
| (2) + [CeCl4(HMPA)2] → (1) + [UCl4(HMPA)2], ΔHrxn = +1.8 kcal mol−1 | (12) |
To further support the above findings, we determined the theoretical bond enthalpy changes (ΔHrxn) for the full, balanced versions of eqn (1)–(6), eqn (7)–(12), by calculating the gas phase geometry optimised structures (all-electron BP86/ZORA/TZP level) of all the constituent components. A solvent continuum was not applied since the solvent for eqn (1)–(6) was benzene, which could reasonably be expected to have systematically minimal interactions with the electropositive species in solution. Experimentally, [ThCl4(THF)3.5] is most likely a separated ion pair formula like related lanthanide triiodides,44 so we approximated it to the molecular analogue [ThCl4(THF)3]. The calculations most likely carry absolute errors of 5–10 kcal mol−1, but, assuming that this is to some extent systematic, the relative errors will reduce to ∼2–5 kcal mol−1. The calculations are thus clear-cut as they independently and correctly reproduce the experimental outcome in every case.
Overall, these exchange reactions demonstrate that thorium(IV) does not displace BIPMTMS from cerium(IV) or uranium(IV) whereas the latter pair do displace BIPMTMS from the former. When cerium(IV) or uranium(IV) derivatives are mixed it is evident that equilibria are established, but the reactions are not clean and the equilibria are disrupted due to the instability of 1. Although some of the products of these reactions are not known, the key point is whether a reaction occurs at all or not. The fact that distinct colour changes are observed, or not, suggests that the carbenes are, or not, transferred since it is the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds that contribute to absorptions in the visible part of the optical spectra for 1 and 2 and the M–ODipp linkages absorb well into the UV-region. Therefore, the conclusion is that thorium(IV) is the most ionic in this context, whereas cerium(IV) and uranium(IV) do exhibit comparable covalency and these observations experimentally support the same theoretical proposition.
C bonds that contribute to absorptions in the visible part of the optical spectra for 1 and 2 and the M–ODipp linkages absorb well into the UV-region. Therefore, the conclusion is that thorium(IV) is the most ionic in this context, whereas cerium(IV) and uranium(IV) do exhibit comparable covalency and these observations experimentally support the same theoretical proposition.
Summary and conclusions
In summary, we have reported the synthesis of 1–3 and on the basis of their characterisation data these complexes can all be described as bona fide formal oxidation state IV complexes. This in turn has provided an opportunity to directly compare the degree of covalency in isostructural cerium, uranium, and thorium carbene complexes. We reiterate that while the bonding of the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C units in these complexes is predominantly ionic, we note a significant covalent contribution to these linkages for cerium and uranium. Significantly, the levels of covalency and f-orbital participation in the M
C units in these complexes is predominantly ionic, we note a significant covalent contribution to these linkages for cerium and uranium. Significantly, the levels of covalency and f-orbital participation in the M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds are remarkably similar for cerium and uranium, but different from thorium which is ionic. Importantly, the similar levels of covalency in the cerium(IV)– and uranium(IV)–carbon multiple bonds in 1 and 2 manifests in more than one type of theoretical treatment (DFT, RASSCF and CASSCF), and most compellingly is supported by experimental exchange reactions that proceed as predicted from the above covalency arguments. It may be that the similar levels of covalency of cerium(IV) and uranium(IV) is a more general effect than currently recognised, but one that is relatively small and so has eluded detection in systems that exhibit minimal covalency. Since the synthesis of cerium(IV) complexes that go beyond simple salts is still in its infancy, and is experimentally challenging, it may be that more examples of cerium(IV) and uranium(IV) complexes containing similar levels of covalency await discovery. At the very least the results presented here provide a basis to question the established exclusive ionic bonding textbook description of the lanthanides in non-zero oxidation states, especially with reference to certain 5f metals.
C bonds are remarkably similar for cerium and uranium, but different from thorium which is ionic. Importantly, the similar levels of covalency in the cerium(IV)– and uranium(IV)–carbon multiple bonds in 1 and 2 manifests in more than one type of theoretical treatment (DFT, RASSCF and CASSCF), and most compellingly is supported by experimental exchange reactions that proceed as predicted from the above covalency arguments. It may be that the similar levels of covalency of cerium(IV) and uranium(IV) is a more general effect than currently recognised, but one that is relatively small and so has eluded detection in systems that exhibit minimal covalency. Since the synthesis of cerium(IV) complexes that go beyond simple salts is still in its infancy, and is experimentally challenging, it may be that more examples of cerium(IV) and uranium(IV) complexes containing similar levels of covalency await discovery. At the very least the results presented here provide a basis to question the established exclusive ionic bonding textbook description of the lanthanides in non-zero oxidation states, especially with reference to certain 5f metals.
Experimental
General
All manipulations were carried out using Schlenk techniques, or an MBraun UniLab glovebox, under an atmosphere of dry nitrogen. Solvents were dried by passage through activated alumina towers and degassed before use or were distilled from calcium hydride. All solvents were stored over potassium mirrors, except for ethers that were stored over activated 4 Å sieves. Deuterated solvent was distilled from potassium, degassed by three freeze–pump–thaw cycles and stored under nitrogen. [Ce(BIPMTMS)(ODipp)2] (1),18 [K(ODipp)], [U(Cl)3(BIPMTMS)Li(THF)2], and [Th(Cl)2(BIPMTMS)] were prepared by published methods.26a,b,451H, 13C, 29Si, and 31P NMR spectra were recorded on a Bruker 400 spectrometer operating at 400.2, 100.6, 79.5, and 162.0 MHz respectively; chemical shifts are quoted in ppm and are relative to TMS (1H, 13C, 29Si) and 85% H3PO4 (31P). FTIR spectra were recorded on a Bruker Tensor 27 spectrometer. UV/Vis/NIR spectra were recorded on a Perkin Elmer Lambda 750 spectrometer. Data were collected in 1 mm path length cuvettes loaded in an MBraun UniLab glovebox and were run versus the appropriate reference solvent. Solution magnetic moments were recorded at room temperature using the Evans method. Static variable-temperature magnetic moment data were recorded in an applied dc field of 0.1 T on a Quantum Design MPMS XL7 superconducting quantum interference device (SQUID) magnetometer using doubly recrystallised powdered samples. Care was taken to ensure complete thermalisation of the sample before each data point was measured and samples were immobilised in an eicosane matrix to prevent sample reorientation during measurements. Diamagnetic corrections were applied for using tabulated Pascal constants and measurements were corrected for the effect of the blank sample holders (flame sealed Wilmad NMR tube and straw) and eicosane matrix. Variable temperature (300–5 K) EPR spectra were measured at X-band (ca. 9 GHz, respectively) on a Bruker Elexsys E580 spectrometer. Polycrystalline samples were sealed under vacuum in 1 mm i.d. silica tubing, and double-contained for EPR by insertion into an X-band silica tube or PTFE sleeve. CHN microanalyses were carried out by Tong Liu at the University of Nottingham. Cerium LIII-edge XANES measurements were performed using a Si(111) double-crystal monochromator on the Rossendorf Beamline at the European Synchrotron Radiation Facility (Grenoble, France). Higher harmonics were rejected by two Si coated mirrors. The spectra were collected using ionisation chambers filled with nitrogen and a 13-element Ge fluorescence detector. The samples were measured at 15 K in a closed-cycle He cryostat. The reference samples spectra of 0.01 M Ce(III) nitrate in H2O and solid CeO2 were measured at room temperature in transmission mode.Preparation of [U(BIPMTMS)(ODipp)2] (2)
THF (15 ml) was added to a precooled (−78 °C) mixture of [U(BIPMTMS)(Cl)3(Li)(THF)2] (1.09 g, 1.0 mmol) and [K(ODipp)] (0.43 g, 2.0 mmol). The resulting brown suspension was allowed to warm to room temperature with stirring over 16 h to afford a brown solution. Volatiles were removed in vacuo and the resulting solid was extracted into toluene. Volatiles were removed in vacuo to afford a brown solid which upon recrystallisation from Et2O (2 ml) at −30 °C afforded 2·Et2O as brown crystals. Yield: 0.69 g, 56%. Anal. calcd for C59H82N2O3P2Si2U: C, 57.91; H, 6.76; N, 2.29%. Found: C, 57.76; H, 6.66; N, 2.33%. 1H NMR (C6D6): δ −18.94 (18H, s, NSi(CH3)3), −3.43 (4H, s, CH(CH3)2), −3.25 (24H, s, CH(CH3)2), 1.23 (6H, s, OCH2CH3), 3.34 (4H, s, OCH2CH3), 6.70 (4H, t, Ar-H), 8.17 (8H, t, Ar-H), 13.40 (2H, t, Dipp-H), 16.07 (4H, d, Dipp-H), 16.48 (Ar-H). 31P{1H} NMR (C6D6): δ −293.42 (UCP2). FTIR ν/cm−1 (Nujol): 1590 (w), 1539 (w), 1403 (w), 1330 (w), 1200 (s), 918 (w), 887 (w), 857 (s), 838 (s), 748 (w), 694 (w), 661 (w). Magnetic moment (Evans method, C6D6, 298 K): μeff = 2.75 μB.Preparation of [Th(BIPMTMS)(ODipp)2] (3)
A solution of [Li2(BIPMTMS)] (0.57 g, 1.0 mmol) in THF (5 ml) was added to a solution of [ThCl4(THF)3.5] (0.63 g, 1.0 mmol) in THF (5 ml) at −78 °C. The pale yellow mixture was stirred at −78 °C for 30 minutes, then was allowed to warm to room temperature with stirring for 2 h. Volatiles were removed in vacuo and DippOK (0.43 g, 2.0 mmol) was added. Toluene (10 ml) was added slowly to the cold (−30 °C) stirring mixture, the resultant mixture was allowed to warm to room temperature with stirring for 1 h. After this time the mixture was filtered, and all volatiles were removed in vacuo. The product was recrystallised from a toluene/hexane mixture to yield 3·0.5(toluene) as colourless crystals. Yield: 0.69 g, 61%. Anal. calcd for C62H80N2O2P2Si2Th: C, 60.27; H, 6.53; N, 2.27%. Found: C, 59.96; H, 6.64; N, 2.45%. 1H NMR (C6D6): δ 0.14 (18H, s, NSi(CH3)3), 1.35 (24H, d, 3JHH = 7.2 Hz, CH(CH3)2), 3.74 (4H, spt, 3JHH = 7.2 Hz, CH(CH3)2), 6.97–7.04 (14H, m, ArH), 7.21 (4H, d, 3JHH = 7.6 Hz, ArH), 7.55–7.61 (8H, m, ArH). 13C{1H} NMR (C6D6): δ 3.18 (s, Si(CH3)3), 24.45 (s, CH(CH3)2), 28.14 (s, CHMe2), 67.04 (t, JPC = 166.7 Hz, Th![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CP2), 120.15, 123.26, 125.66, 128.53, 129.29, 130.11 (ArC), 131.36 (d, 3JPC = 6.4 Hz, Cmeta of P–Ph), 131.43 (d, 3JPC = 5.5 Hz, Cmeta of P–Ph), 136.94 (s, ArC), 139.01 (d, JPC = 48.2 Hz, Cipso of P–Ph), 139.49 (d, JPC = 47.4 Hz, Cipso of P–Ph), 161.26 (s, ArC). 31P{1H} NMR (C6D6) δ 4.65 (s). 29Si{1H} NMR (C6D6) δ −7.20 (d, 2JPSi = 3.11 Hz), −7.24 (d, 2JPSi = 3.07 Hz). FTIR ν/cm−1 (Nujol): 1589 (w), 1325 (w), 1260 (s), 1197 (m), 1100 (br, s), 1095 (br, s), 1042 (m), 1023 (m), 887 (w), 856 (m), 800 (m), 726 (m), 609 (m).
CP2), 120.15, 123.26, 125.66, 128.53, 129.29, 130.11 (ArC), 131.36 (d, 3JPC = 6.4 Hz, Cmeta of P–Ph), 131.43 (d, 3JPC = 5.5 Hz, Cmeta of P–Ph), 136.94 (s, ArC), 139.01 (d, JPC = 48.2 Hz, Cipso of P–Ph), 139.49 (d, JPC = 47.4 Hz, Cipso of P–Ph), 161.26 (s, ArC). 31P{1H} NMR (C6D6) δ 4.65 (s). 29Si{1H} NMR (C6D6) δ −7.20 (d, 2JPSi = 3.11 Hz), −7.24 (d, 2JPSi = 3.07 Hz). FTIR ν/cm−1 (Nujol): 1589 (w), 1325 (w), 1260 (s), 1197 (m), 1100 (br, s), 1095 (br, s), 1042 (m), 1023 (m), 887 (w), 856 (m), 800 (m), 726 (m), 609 (m).
Computational details
Unrestricted and restricted geometry optimisations were performed as appropriate for full models of 1, 2, and 3 and the components of the exchange reactions using coordinates derived from their X-ray crystal structures. No constraints were imposed on the structures during the geometry optimisations. The calculations were performed using the Amsterdam Density Functional (ADF) suite version 2010.01.46,47 The DFT geometry optimisations employed Slater type orbital (STO) triple-ζ-plus polarisation all-electron basis sets (from the ZORA/TZP database of the ADF suite). Scalar relativistic approaches were used within the ZORA Hamiltonian for the inclusion of relativistic effects and the local density approximation (LDA) with the correlation potential due to Vosko et al.48 was used in all of the calculations. Gradient corrections were performed using the functionals of Becke49 and Perdew.50 Following geometry optimisation a single point energy (SPE) calculation was performed. MOLEKEL51 was used to prepare the three-dimensional plot of the electron density. Natural Bond Order (NBO) analyses were carried out with NBO 5.052 since this method is well suited to describing covalency effects in molecules.35,52 Optimisations of the L–H bonds in hydrogen terminated truncated complexes were performed using version 6.4 of the TURBOMOLE software package,53 employing the PBE functional,54 based on the generalised gradient approximation (GGA). Ahlrichs basis sets55 of polarised triple-ζ quality (def-TZVP for Ce, Th, U; def2-TZVP for all other atoms) were used for these partial optimisations. Total electron densities were obtained via single point energy (SPE) calculations, replacing the basis sets of the metal ions with the segmented all-electron relativistically contracted (SARC) basis sets,56 again of polarised triple-ζ quality. In these SPE calculations, scalar relativistic effects were incorporated via the 2nd order Douglas–Kroll–Hess Hamiltonian.57,58 Correlated electron wavefunctions of the truncated systems were obtained by employing the restricted-active-space and complete active space self-consistent-field (RASSCF/CASSCF) methodologies59 using version 7.6 of the MOLCAS software package.60,61 In these calculations, all-electron ANO-RCC basis sets62–64 of approximate polarised triple-ζ quality were employed, with scalar relativistic effects again incorporated via the 2nd order Douglas–Kroll–Hess Hamiltonian. Topological and integrated atomic properties, obtained using the quantum theory of atoms in molecules (QTAIM), were performed using version 13.11.04 of the AIMAll software package.65Acknowledgements
We are grateful to the Royal Society, the UK Engineering and Physical Sciences Research Council, the Marie Curie IIF Scheme, the European Research Council, The University of Nottingham, The University of Manchester, the UK National Nuclear Laboratory, and COST Action CM1006 for generous support and funding, and the National Service for Computational Chemistry Software for access to the Columbus HPC facility.Notes and references
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, Ithaca, New York, 1939 Search PubMed.
- S. Cotton, Lanthanide and Actinide Chemistry, John Wiley & Sons Ltd, Chichester, UK, 2006 Search PubMed.
- W. J. Evans, Inorg. Chem., 2007, 46, 3435 CrossRef CAS PubMed.
- (a) S. A. Kozimor, P. Yang, E. R. Batista, K. S. Boland, C. J. Burns, D. L. Clark, S. D. Conradson, R. L. Martin, M. P. Wilkerson and L. W. Wolfsberg, J. Am. Chem. Soc., 2009, 131, 12125 CrossRef CAS PubMed; (b) S. G. Minasian, J. M. Keith, E. R. Batista, K. S. Boland, D. L. Clark, S. D. Conradson, S. A. Kozimor, R. L. Martin, D. E. Schwarz, D. K. Shuh, G. L. Wagner, M. P. Wilkerson, L. E. Wolfsberg and P. Yang, J. Am. Chem. Soc., 2012, 134, 5586 CrossRef CAS PubMed; (c) M. B. Jones, A. J. Gaunt, J. C. Gordon, N. Kaltsoyannis, M. P. Neu and B. L. Scott, Chem. Sci., 2013, 4, 1189 RSC; (d) W. W. Lukens, N. M. Edelstein, N. Magnani, T. W. Hayton, S. Fortier and L. A. Seaman, J. Am. Chem. Soc., 2013, 135, 10742 CrossRef CAS PubMed; (e) M. J. Polinski, E. B. Garner III, R. Maurice, N. Planas, J. T. Stritzinger, T. G. Parker, J. N. Cross, T. D. Green, E. V. Alekseev, S. M. van Cleve, W. Depmeier, L. Gagliardi, M. Shatruk, K. L. Knappenberger, G. Liu, S. Skanthakumar, L. Soderholm, D. A. Dixon and T. E. Albrecht-Schmitt, Nat. Chem., 2014, 6, 387 CrossRef CAS PubMed; (f) M. W. Löble, J. M. Keith, A. B. Altman, S. C. E. Stieber, E. R. Batista, K. S. Boland, S. D. Conradson, D. L. Clark, J. L. Pacheco, S. A. Kozimor, R. L. Martin, S. G. Minasian, A. C. Olson, B. L. Scott, D. K. Shuh, T. Tyliszczak, M. P. Wilkerson and R. A. Zehnder, J. Am. Chem. Soc., 2015, 137, 2506 CrossRef PubMed; (g) S. K. Cary, M. Vasiliu, R. E. Baumbach, J. T. Stritzinger, T. D. Green, K. Diefenbach, J. N. Cross, K. L. Knappenberger, G. Liu, M. A. Silver, A. E. DePrince, M. J. Polinski, S. M. van Cleve, J. A. House, N. Kikugawa, A. Gallagher, A. A. Arico, D. A. Dixon and T. E. Albrecht-Schmitt, Nat. Commun., 2015, 6, 6827 CrossRef CAS PubMed.
- R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- (a) I. Castro-Rodríguez and K. Meyer, Chem. Commun., 2006, 1353 RSC; (b) M. Ephritikhine, Dalton Trans., 2006, 2501 RSC; (c) O. P. Lam, C. Anthon and K. Meyer, Dalton Trans., 2009, 9677 RSC; (d) T. W. Hayton, Dalton Trans., 2010, 39, 1145 RSC; (e) H. S. La Pierre and K. Meyer, Inorg. Chem., 2013, 52, 529 CrossRef CAS PubMed; (f) M. Ephritikhine, Organometallics, 2013, 32, 2464 CrossRef CAS; (g) M. B. Jones and A. J. Gaunt, Chem. Rev., 2013, 113, 1137 CrossRef CAS PubMed; (h) B. M. Gardner and S. T. Liddle, Eur. J. Inorg. Chem., 2013, 3753 CrossRef CAS; (i) H. S. La Pierre and K. Meyer, Prog. Inorg. Chem., 2014, 58, 303 CrossRef CAS; (j) S. T. Liddle, Angew. Chem., Int. Ed., 2015, 54, 8604 CrossRef CAS PubMed.
- (a) L. S. Natrajan, Coord. Chem. Rev., 2012, 256, 1583 CrossRef CAS; (b) J. L. Brown, S. Fortier, R. A. Lewis, G. Wu and T. W. Hayton, J. Am. Chem. Soc., 2012, 134, 15468 CrossRef CAS PubMed; (c) D. M. King, J. McMaster, F. Tuna, E. J. L. McInnes, W. Lewis, A. J. Blake and S. T. Liddle, J. Am. Chem. Soc., 2014, 136, 5619 CrossRef CAS PubMed; (d) B. M. Gardner, G. Balázs, M. Scheer, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2014, 53, 4484 CrossRef CAS PubMed; (e) B. M. Gardner, G. Balázs, M. Scheer, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Nat. Chem., 2015, 7, 582 CrossRef CAS PubMed.
- J.-C. G. Bünzli and S. V. Eliseeva, in Lanthanide Luminescence; Springer Series on Fluorescence, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, vol. 7, p. 1 Search PubMed.
- (a) R. J. Long, Can. J. Res., 1936, 14a, 127 CrossRef; (b) G. Blasse and A. Bril, J. Chem. Phys., 1967, 47, 5139 CrossRef CAS; (c) W. K. Kot, PhD Thesis, University of California Berkeley, 1991.
- (a) F. G. N. Cloke, Chem. Soc. Rev., 1993, 22, 17 RSC; (b) S. G. Minasian, J. L. Krinsky and J. Arnold, Chem.–Eur. J., 2011, 17, 12234 CrossRef CAS PubMed . There is also mounting but isolated evidence that cerium(IV) is a special case where f-orbital participation in cerium–ligand bonding has been identified. See ref. 19n below for a representative example.
- H. H. Dam, D. N. Reinhoudt and W. Verboom, Chem. Soc. Rev., 2007, 36, 367 RSC . A pertinent example to this work is a study that reveals discrimination of binding of an N-heterocyclic carbene to uranium(III) over cerium(III), which suggests greater covalent bonding for the former, see: T. Mehdoui, J.-C. Berthet, P. Thuéry and M. Ephritikhine, Chem. Commun., 2005, 2860 RSC.
- (a) A. Kerridge, Dalton Trans., 2013, 42, 16428 RSC; (b) A. Kerridge, RSC Adv., 2014, 4, 12078 RSC; (c) S. G. Minasian, J. M. Keith, E. R. Batista, K. S. Boland, D. L. Clark, S. A. Kozimor, R. L. Martin, D. K. Shuh and T. Tyliszczak, Chem. Sci., 2014, 5, 351 RSC.
- (a) A. J. Gaunt, S. D. Reilly, A. E. Enriquez, B. L. Scott, J. A. Ibers, P. Sekar, K. I. M. Ingram, N. Kaltsoyannis and M. P. Neu, Inorg. Chem., 2008, 47, 29 CrossRef CAS PubMed; (b) K. I. M. Ingram, M. J. Tassell, A. J. Gaunt and N. Kaltosyannis, Inorg. Chem., 2008, 47, 7824 CrossRef CAS PubMed; (c) P. L. Arnold, Z. R. Turner, N. Kaltsoyannis, P. Pelekanaki, R. M. Bellabarba and R. P. Tooze, Chem.–Eur. J., 2010, 16, 9623 CrossRef CAS PubMed; (d) T. Arliguie, L. Belkhiri, S. E. Bouaoud, P. Thuery, C. Villiers, A. Boucekkine and M. Ephritikhine, Inorg. Chem., 2009, 48, 221 CrossRef CAS PubMed.
- K. S. Thanthiriwatte, M. Vasiliu, S. R. Battey, Q. Lu, K. A. Peterson, L. Andrews and D. A. Dixon, J. Phys. Chem. A, 2015, 119, 5790 CrossRef CAS PubMed.
- (a) B. O. Roos and P. Pyykkö, Chem.–Eur. J., 2010, 16, 270 CrossRef CAS PubMed; (b) W. Xu, X. Jin, M. Chen, P. Pyykkö, M. Zhou and J. Li, Chem. Sci., 2012, 3, 1548 RSC.
- (a) M. L. Neidig, D. L. Clark and R. L. Martin, Coord. Chem. Rev., 2013, 257, 394 CrossRef CAS; (b) N. Kaltsoyannis, Inorg. Chem., 2013, 52, 3407 CrossRef CAS PubMed.
- (a) G. R. Giesbrecht and J. C. Gordon, Dalton Trans., 2004, 2387 RSC; (b) T. Cantat, N. Mézailles, A. Auffrant and P. Le Floch, Dalton Trans., 2008, 1957 RSC; (c) S. T. Liddle, D. P. Mills and A. J. Wooles, Chem. Soc. Rev., 2011, 40, 2164 RSC; (d) O. T. Summerscales and J. C. Gordon, RSC Adv., 2013, 3, 6682 RSC; (e) J. Kratsch and P. W. Roesky, Angew. Chem., Int. Ed., 2014, 53, 376 CrossRef CAS PubMed.
- M. Gregson, E. Lu, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2013, 52, 13016 CrossRef CAS PubMed.
- (a) W. J. Evans, T. J. Deming, J. M. Olofson and J. W. Ziller, Inorg. Chem., 1989, 28, 4027 CrossRef CAS; (b) C. Morton, N. W. Alcock, M. R. Lees, I. J. Munslow, C. J. Sanders and P. Scott, J. Am. Chem. Soc., 1999, 121, 11255 CrossRef CAS; (c) O. Eisenstein, P. B. Hitchcock, A. G. Hulkes, M. F. Lappert and L. Maron, Chem. Commun., 2001, 1560 RSC; (d) Y. K. Gun'ko, S. D. Elliott, P. B. Hitchcock and M. F. Lappert, J. Chem. Soc., Dalton Trans., 2002, 1852 RSC; (e) P. B. Hitchcock, A. G. Hulkes and M. F. Lappert, Inorg. Chem., 2004, 43, 1031 CrossRef CAS PubMed; (f) P. B. Hitchcock, M. F. Lappert and A. V. Protchenko, Chem. Commun., 2006, 3546 RSC; (g) E. M. Broderick and P. L. Diaconescu, Inorg. Chem., 2009, 48, 4701 CrossRef CAS PubMed; (h) P. Dröse, A. R. Crozier, S. Lashkari, J. Gottfriedsen, S. Blaurock, C. G. Hrib, C. Maichle-Mössmer, C. Schädle, R. Anwander and F. T. Edelmann, J. Am. Chem. Soc., 2010, 132, 14046 CrossRef PubMed; (i) M. P. Coles, P. B. Hitchcock, A. V. Khvostov, M. F. Lappert, Z. Li and A. V. Protchenko, Dalton Trans., 2010, 39, 6780 RSC; (j) E. M. Broderick, P. S. Thuy-Boun, N. Guo, C. S. Vogel, J. Sutter, J. T. Miller, K. Meyer and P. L. Diaconescu, Inorg. Chem., 2011, 50, 2870 CrossRef CAS PubMed; (k) E. M. Broderick, N. Guo, T. Wu, C. S. Vogel, C. Xu, J. Sutter, J. T. Miller, K. Meyer, T. Cantat and P. L. Diaconescu, Chem. Commun., 2011, 47, 9897 RSC; (l) J. R. Robinson, P. J. Carroll, P. J. Walsh and E. J. Schelter, Angew. Chem., Int. Ed., 2012, 51, 10159 CrossRef CAS PubMed; (m) B. D. Mahoney, N. A. Piro, P. J. Carroll and E. J. Schelter, Inorg. Chem., 2013, 52, 5970 CrossRef CAS PubMed; (n) J. A. Bogart, A. J. Lewis, S. A. Medling, N. A. Piro, P. J. Carroll, C. H. Booth and E. J. Schelter, Inorg. Chem., 2013, 52, 11600 CrossRef CAS PubMed; (o) J. R. Robinson, Z. Gordon, C. H. Booth, P. J. Carroll, P. J. Walsh and E. J. Schelter, J. Am. Chem. Soc., 2013, 135, 19016 CrossRef CAS PubMed; (p) A. R. Crozier, A. M. Bienfait, C. Maichle-Mössmer, K. W. Törnroos and R. Anwander, Chem. Commun., 2013, 49, 87 RSC; (q) U. J. Williams, B. D. Mahoney, A. J. Lewis, P. T. DeGregorio, P. J. Carroll and E. J. Schelter, Inorg. Chem., 2013, 52, 4142 CrossRef CAS PubMed; (r) D. Werner, G. B. Deacon, P. C. Junk and R. Anwander, Chem.–Eur. J., 2014, 20, 4426 CrossRef CAS PubMed; (s) U. J. Williams, J. R. Robinson, A. J. Lewis, P. J. Carroll, P. J. Walsh and E. J. Schelter, Inorg. Chem., 2014, 53, 27 CrossRef CAS PubMed; (t) H. B. Lee, J. A. Bogart, P. J. Carroll and E. J. Schelter, Chem. Commun., 2014, 50, 5361 RSC.
- (a) A. Streitwieser Jr, S. A. Kinsley, J. T. Rigsbee, I. L. Fragala and E. Ciliberto, J. Am. Chem. Soc., 1985, 107, 7786 CrossRef; (b) W. J. Evans, T. J. Deming and J. W. Ziller, Organometallics, 1989, 8, 1581 CrossRef CAS; (c) A. Streitwieser Jr, S. A. Kinsley, C. H. Jenson and J. T. Rigsbee, Organometallics, 2004, 23, 5169 CrossRef; (d) C. H. Booth, M. D. Walter, M. Daniel, W. W. Lukens and R. A. Andersen, Phys. Rev. Lett., 2005, 95, 267202 CrossRef CAS PubMed; (e) I. J. Casely, S. T. Liddle, A. J. Blake, C. Wilson and P. L. Arnold, Chem. Commun., 2007, 5037 RSC; (f) M. D. Walter, C. H. Booth, W. W. Lukens and R. A. Andersen, Organometallics, 2009, 28, 698 CrossRef CAS.
- F. M. A. Sroor and F. T. Edelmann, The Rare Earth Elements Fundamentals and Applications, ed. D. A. Atwood, John Wiley & Sons, Chichester, 2012, pp. 313 and 321 Search PubMed.
- D. P. Mills, F. Moro, J. McMaster, J. van Slageren, W. Lewis, A. J. Blake and S. T. Liddle, Nat. Chem., 2011, 3, 454 CAS.
- J. R. Levin, W. L. Dorfner, P. J. Carroll and E. J. Schelter, Chem. Sci., 2015, 6, 6925 RSC.
- D. L. Clark, J. C. Gordon, P. J. Hay and R. Poli, Organometallics, 2005, 24, 5747 CrossRef CAS.
- M. Yamada, T. Nakahodo, T. Wakahara, T. Tsuchiya, Y. Maeda, T. Akasaka, M. Kako, K. Yoza, E. Horn, N. Mizorogi, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 2005, 127, 14570 CrossRef CAS PubMed.
- (a) O. J. Cooper, D. P. Mills, J. McMaster, F. Moro, E. S. Davies, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2011, 50, 2383 CrossRef CAS PubMed; (b) G. Ma, M. J. Ferguson, R. McDonald and R. G. Cavell, Inorg. Chem., 2011, 50, 6500 CrossRef CAS PubMed; (c) D. P. Mills, O. J. Cooper, F. Tuna, E. J. L. McInnes, E. S. Davies, J. McMaster, F. Moro, W. Lewis, A. J. Blake and S. T. Liddle, J. Am. Chem. Soc., 2012, 134, 10047 CrossRef CAS PubMed; (d) O. J. Cooper, D. P. Mills, J. McMaster, F. Tuna, E. J. L. McInnes, W. Lewis, A. J. Blake and S. T. Liddle, Chem.–Eur. J., 2013, 19, 7071 CrossRef CAS PubMed; (e) E. Lu, O. J. Cooper, J. McMaster, F. Tuna, E. J. L. McInnes, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2014, 53, 6696 CrossRef CAS PubMed; (f) E. Lu, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2014, 53, 9356 CrossRef CAS PubMed.
- D. R. Kindra and W. J. Evans, Chem. Rev., 2014, 114, 8865 CrossRef CAS PubMed.
- J. J. Le Roy, I. Korobkov, J. E. Kim, E. J. Schelter and M. Murugesu, Dalton Trans., 2014, 43, 2737 RSC.
- C. Hennig, A. Ikeda-Ohno, W. Kraus, S. Weiss, P. Pattison, H. Emerich, P. M. Abdala and A. C. Scheinost, Inorg. Chem., 2013, 52, 11734 CrossRef CAS PubMed.
- (a) A. Bianconi, A. Marcelli, M. Tomellini and I. Davoli, J. Magn. Magn. Mater., 1985, 47–48, 209 CrossRef CAS; (b) A. Bianconi, A. Marcelli, H. Dexpert, A. Kotani, T. Jo and J. Petiau, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 806 CrossRef CAS; (c) G. Kaindl, G. K. Wertheim and G. Schmiester, Phys. Rev., 1987, 58, 606 CAS; (d) G. Kaindl, G. Schmiester, E. Sampathkumaran and P. Wachter, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 10174 CrossRef CAS; (e) A. M. Shahin, F. Grandjean, G. J. Long and T. P. Schuman, Chem. Mater., 2005, 17, 315 CrossRef CAS.
- (a) C. H. Booth, M. D. Walter, M. Daniel, W. W. Lukens and R. A. Andersen, Phys. Rev. Lett., 2005, 95, 267202 CrossRef CAS PubMed; (b) M. D. Walter, C. H. Booth, W. W. Lukens and R. A. Andersen, Organometallics, 2009, 28, 698 CrossRef CAS.
- Although the spectrum of 1 is saturated the ratio of peaks for 1 is unequivocally more like CeO2 than cerocene derivatives.
- (a) A. Kotani, H. Ogasawara, K. Okada, B. T. Thole and G. A. Sawatzky, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 65 CrossRef CAS; (b) A. Kotani, K. O. Kvashnina, S. M. Butorin and P. Glatzel, Eur. Phys. J. B, 2012, 85, 257 CrossRef.
- (a) T. Hanyu, H. Ishii, M. Yanagihara, T. Kamada, T. Miyahara, H. Kato, K. Naito, S. Suzuki and T. Ishii, Solid State Commun., 1985, 56, 381 CrossRef CAS; (b) F. Marabelli and P. Wachter, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 36, 1238 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899 CrossRef CAS.
- P.-Å. Malmqvist, A. Rendell and B. O. Roos, J. Phys. Chem., 1990, 94, 5477 CrossRef CAS.
- R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Oxford University Press, 1990 Search PubMed.
- (a) A. Kerridge, R. Coates and N. Kaltsoyannis, J. Phys. Chem. A, 2009, 133, 2896 CrossRef PubMed; (b) A. Kerridge and N. Kaltsoyannis, C. R. Chim., 2010, 13, 853 CrossRef CAS; (c) R. Coates, M. Coreno, M. DeSimone, J. C. Green, N. Kaltsoyannis, A. Kerridge, A. Narband and A. Sella, Dalton Trans., 2009, 5943 RSC.
- X. Fradera, M. A. Austen and R. F. W. Bader, J. Phys. Chem. A, 1999, 103, 304 CrossRef CAS.
- (a) M. J. Tassell and N. Kaltsoyannis, Dalton Trans., 2010, 39, 6576 RSC; (b) I. Kirker and N. Kaltsoyannis, Dalton Trans., 2011, 40, 124 RSC.
- V. Veryazov, P. Å. Malmqvist and B. O. Roos, Int. J. Quantum Chem., 2011, 111, 3329 CrossRef CAS.
- (a) M. Dolg, P. Fulde, W. Küchle, C.-S. Neumann and H. Stoll, J. Chem. Phys., 1991, 94, 3011 CrossRef CAS; (b) O. Mooßen and M. Dolg, Chem. Phys. Lett., 2014, 594, 47 CrossRef.
- H. Werner, Angew. Chem., Int. Ed., 2012, 51, 6052 CrossRef CAS PubMed.
- K. Izod, S. T. Liddle and W. Clegg, Inorg. Chem., 2004, 43, 214 CrossRef CAS PubMed.
- T. J. Boyle, N. L. Andrews, M. A. Rodriguez, C. Campana and T. Yiu, Inorg. Chem., 2003, 42, 5357 CrossRef CAS PubMed.
- C. Fonseca-Guerra, J. G. Snijders, G. Te Velde and E. J. Baerends, Theor. Chem. Acc., 1998, 99, 391 Search PubMed.
- G. Te Velde, F. M. Bickelhaupt, S. J. Van Gisbergen, A. C. Fonseca-Guerra, E. J. Baerends, J. G. Snijders and T. J. Ziegler, Comput. Chem., 2001, 22, 931 CrossRef CAS.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822 CrossRef.
- F. M. Bickelhaupt, N. M. Van Nibbering, E. M. Wezenbeek and E. J. Baerends, J. Phys. Chem., 1992, 96, 4864 CrossRef CAS.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales and F. Weinhold, NBO 5.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2001, http://www.chem.wisc.edu/%7Enbo5 Search PubMed.
- R. Ahlrichs, M. K. Armbruster, M. Bär and H.-P. Baron, TURBOMOLE V 6.4, 2012 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297 RSC.
- D. A. Pantazis and F. Neese, J. Chem. Theory Comput., 2011, 7, 677 CrossRef CAS.
- M. Douglas and N. Kroll, Ann. Phys., 1974, 155, 89 Search PubMed.
- B. Hess, Phys. Rev. A, 1986, 33, 3742 CrossRef CAS.
- P.-Å. Malmqvist, A. Rendell and B. O. Roos, J. Phys. Chem., 1990, 94, 5478 CrossRef.
- G. Karlström, R. Lindh, P.-Å. Malmqvist, B. O. Roos, U. Ryde, V. Veryazov, P.-O. Widmark, M. Cossi, B. Schimmelpfennig, P. Neogrady and L. Seijo, Comput. Mater. Sci., 2003, 28, 222 CrossRef.
- F. Aquilante, L. D. E. Vico, N. Ferré, G. Ghigo, P.-Å. Malmqvist, P. Neogrády, T. B. Pedersen, M. P. Nák, M. Reiher, B. O. Roos, L. Serrano-andrés, M. Urban, V. Veryazov and R. Lindh, J. Comput. Chem., 2010, 31, 224 CrossRef CAS PubMed.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov and P.-O. Widmark, J. Phys. Chem. A, 2004, 108, 2851 CrossRef CAS.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov, P.-O. Widmark and A. C. Borin, J. Phys. Chem. A, 2008, 112, 11431 CrossRef CAS PubMed.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov and P.-O. Widmark, Chem. Phys. Lett., 2005, 409, 295 CrossRef CAS.
- T. A. Keith, AIMAll (Version 13.11.04), TK Gristmill Software, Overl. Park KS, USA, 2013 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental considerations, crystallographic, and computational details for compounds 2 and 3. CCDC 938905 and 938904. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc00278a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |