Open Access Article
Open Access ArticleHigh conductivity Ag-based metal organic complexes as dopant-free hole-transport materials for perovskite solar cells with high fill factors†
Yong
Hua‡
a,
Bo
Xu‡
a,
Peng
Liu
b,
Hong
Chen
a,
Haining
Tian
d,
Ming
Cheng
a,
Lars
Kloo
b and
Licheng
Sun
*ac
aOrganic Chemistry, Center of Molecular Devices, Department of Chemistry, School of Chemical Science and Engineering, KTH Royal Institute of Technology, Teknikringen 30, SE-10044, Stockholm, Sweden. E-mail: lichengs@kth.se; Fax: +46-8-791-2333
bApplied Physical Chemistry, Department of Chemistry, School of Chemical Science and Engineering, KTH Royal Institute of Technology, Teknikringen 30, SE-10044, Stockholm, Sweden
cState Key Laboratory of Fine Chemicals, DUT-KTH Joint Research Centre on Molecular Devices, Dalian University of Technology (DUT), 116024 Dalian, China
dPhysical Chemistry, Department of Chemistry-Ångström Laboratory, Uppsala University (UU), SE-751 20 Uppsala, Sweden. E-mail: haining.tian@kemi.uu.se
First published on 15th December 2015
Abstract
Hole-transport materials (HTMs) play an important role as hole scavenger materials in the most efficient perovskite solar cells (PSCs). Here, for the first time, two Ag-based metal organic complexes (HA1 and HA2) are employed as a new class of dopant-free hole-transport material for application in PSCs. These HTMs show excellent conductivity and hole-transport mobility. Consequently, the devices based on these two HTMs exhibit unusually high fill factors of 0.76 for HA1 and 0.78 for HA2, which are significantly higher than that obtained using spiro-OMeTAD (0.69). The cell based on HA1-HTM in its pristine form achieved a high power conversion efficiency of 11.98% under air conditions, which is comparable to the PCE of the cell employing the well-known doped spiro-MeOTAD (12.27%) under the same conditions. More importantly, their facile synthesis and purification without using column chromatography makes these new silver-based HTMs highly promising for future commercial applications of PSCs. These results provide a new way to develop more low-cost and high conductivity metal-complex based HTMs for efficient PSCs.
The hybrid organic–inorganic perovskite solar cells (PSCs) have garnered considerable interest because of their unique combination of photophysical and chemical properties, such as excellent light absorption, charge-carrier conductivity, a low-temperature solution-fabrication process and high conversion efficiency.1–6 In 2009, the [CH3NH3]PbI3-based perovskite was initially employed as a light sensitizer in the conventional tri-iodide/iodine liquid electrolyte-based dye-sensitized solar cells (DSCs), resulting in a power conversion efficiency (PCE) of 3.8%.7 Later, in 2012, the efficiency was further increased up to 9.7% by replacing the liquid electrolyte with the p-type solid-state hole-transport material (HTM) spiro-OMeTAD in all-solid-state PSCs.8 Since then, impressive improvements have been achieved regarding the photovoltaic performance and stability of this type of device. Typically, a porous PSC is composed of a fluorine-doped tin oxide (FTO)-covered glass substrate with a thin layer of dense titanium oxide (TiO2) as a blocking layer, a mesoporous semiconductor film (usually TiO2), the mesoporous film is successively decorated with a layer of perovskite crystal, a HTM layer and finally a metal counter electrode. In this kind of device configuration, the electrical and optical properties of HTMs can efficiently separate and extract the photogenerated holes from the perovskite absorber to the back contact metal, which significantly affect the performance of the devices.9–15 Consequently, extensive research has been devoted to the development of new and efficient HTMs for solid-state PSCs, including inorganic semiconductors,16,17 organic small molecule hole conductors18–28 and conducting polymers.29–32 Although a variety of organic HTMs have been developed for PSCs, the most common HTM spiro-OMeTAD to date gives rise to the best device performance. However, the multistep synthesis with low yield and time-consuming purification of the spiro-OMeTAD limit its potential commercialization in photovoltaics due to the resulting high cost. Moreover, like other organic HTMs, spiro-OMeTAD suffers from low intrinsic conductivity (∼10−5 S cm−2) in its pristine form; chemical p-type dopants, such as Ag-bis(trifluoromethanesulfonyl)imide (Ag-TFSI)33 and cobalt complexes (FK102),34 have proven to enhance its conductivity and therefore improve the efficiencies of PSCs. However, the aforementioned doping strategy requires much optimization of the doping conditions, such as the solvents and doping concentrations. Therefore, it is a highly relevant challenge to develop dopant-free HTMs with simpler synthetic routes and comparable device performance to qualify as potential alternatives to spiro-OMeTAD for PSC applications.
Herein, two novel Ag-based metal organic complexes are introduced as efficient dopant-free HTMs (HA1 and HA2) for CH3NH3PbI3-based PSCs (Fig. 1). These HTMs were synthesized through facile reactions in high yields up to 85%. Furthermore, their simple and quick purification without having to use column chromatography makes these HTMs very promising for commercial application in PSCs. The metal–organic complex-based HTMs show excellent conductivity and hole-transport ability. The device based on HA1-HTM in its pristine form renders PSCs with a PCE of 11.98% with a high FF of 0.76 under ambient atmosphere conditions, which is comparable to the PCE of the cell employing the well-known doped spiro-OMeTAD (12.27% with a FF of 0.69) under the same conditions. The device based on HA2-HTM achieved a PCE of 10.79% with an excellent FF of 0.78. As alternatives to spiro-OMeTAD, this work is the first report on the use of metal–organic complex as dopant-free HTMs that can significantly increase conductivity and hole mobility, resulting in a high fill factor for PSCs.
The molecular structures of the two new HTMs, HA1 and HA2, are shown in Fig. 1 and the synthetic route to the HTMs is shown in Scheme S1.† The final products were synthesized through a simple process in only two steps. Briefly, 4-methoxy-N-(4-methoxyphenyl)-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline was coupled with 4-bromopyridine or 4-bromo-7-(pyridin-4-yl)benzo[c][1,2,5]thiadiazole in a Suzuki–Miyaura cross-coupling reaction to obtain 1 in 88% yield and 2 in 85% yield, respectively. The reactions of 1 or 2 with silver bis(trifluoromethylsulphonyl)imide (AgTFSI) afforded HA1 and HA2 in quantitative yields.
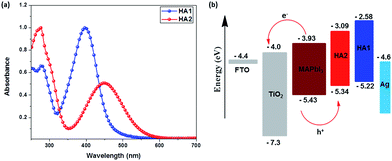
The UV-vis absorption spectra of these two HTMs measured in CH2Cl2 solution are displayed in Fig. 2a and all data are summarized in Table 1. The absorption spectra of the HTMs HA1 and HA2 display a maximum absorption peak at 397 nm and 448 nm, respectively. It should be mentioned that HA2 shows a significant bathochromic shift as compared to that of HA1, which can be attributed to the introduction of the electron-withdrawing benzothiadiazole unit into the molecular framework, leading to an enhancement of the intramolecular charge transfer (ICT) from donor to acceptor.
| HTM | λ max [nm] | E 0–0 [eV] | E HOMO [eV] | E LUMO [eV] | Hole mobility [cm2 V−1 s−1] | Conductivity [S cm−1] |
|---|---|---|---|---|---|---|
| a Absorption maximum in 1 × 10−5 mol L−1 CH2Cl2 solution. b E 0–0 was determined from the intersection of the normalized absorption and emission spectra. c 0.1 M of tetrabutylammonium hexafluorophosphate (n-Bu4NPF6) in CH2Cl2 solution as electrolyte; Ag/0.01 M AgNO3 electrode (acetonitrile as solvent) as the reference electrode; a glassy carbon disk (diameter 3 mm) as the working electrode; a platinum wire as the counter electrode. Scan rate: 50 mV s−1. Each measurement was calibrated with Fc. EFc1/2 = 0.20 V. EHOMO = −5.1 − (E1/2 − EFc1/2). d E LUMO = EHOMO + E0–0. | ||||||
| HA1 | 397 | 2.64 | −5.22 | −2.58 | 6.49 × 10−4 | 1.05 × 10−3 |
| HA2 | 448 | 2.25 | −5.34 | −3.09 | 8.38 × 10−4 | 1.78 × 10−3 |
To evaluate the possibility of hole transfer from the perovskite to the HTM, the electronic properties of HA1 and HA2 (Fig. S2†) were determined by cyclic voltammetry in CH2Cl2 solutions containing 0.1 mM tetrabutylammonium hexafluorophosphate (TBAPF6) as electrolyte in a three-electrode system. The data are presented in Table 1. The HOMO energy level was estimated according to the literature (assuming that the energy level of Fc+/Fc = −5.1 eV).35,36 The HOMO energy levels of HA1 and HA2 are estimated to −5.22 and −5.34 eV, respectively. These values lie above that of CH3NH3PbI3 (−5.43 eV), which should ensure sufficient driving force for the hole injection from CH3NH3PbI3 into the silver-based HTMs. The HOMO energy level of HA2 is negatively shifted by 120 mV as compared to that of HA1 due to the introduction of the electron-deficient benzothiadiazole unit. The deeper HOMO energy level indicates that HA2 may be expected to generate a higher Voc than HA1 in PSCs, since the Voc of PSCs theoretically is mainly governed by the energy difference between the quasi-Fermi levels of the electrons in the TiO2 and the HOMO energy level of the HTM used. Besides, the LUMO levels of HA1 (−2.58 eV) and HA2 (−3.09 eV) are higher than that of CH3NH3PbI3 (−3.93 eV), which should guarantee blocking of the electron transport from CH3NH3PbI3 to Ag counter electrode and hence suppress the carrier recombination.37–39
To gain insights into the geometric and electronic properties of these new HTMs, density functional calculations (DFT) were performed using the Gaussian 09 program package at the B3LYP/6-31G(d)* level of theory. In optimal structural conformations shown in Fig. S3,† the electron density distribution of the HOMOs of the HA1 and HA2 are mainly located at the donor triphenylamine part, whereas the electron density distributions of the LUMOs are primarily located at the acceptor units (pyridine unit for HA1 and benzothiadiazole and pyridine units for HA2) and to a small extent at the neighboring benzene ring. Hence, the substantial overlapping between HOMO and LUMO orbitals guarantee the prerequisites for the fast formation of neutral excitons and hole-transfer transitions.40 Consequently, fast hole transport may suppress charge-recombination at the TiO2/HTM interface.
The hole mobility and conductivity are significant parameters for estimating the potential usability of new HTMs in PSC applications. Here, the hole mobility of the HTMs were determined by using space-charge-limited currents (SCLCs) following previous reports35,36 and the conductivity was determined by using a two-contact electrical conductivity setup.41,42 The detailed results are presented in Table 1. Obviously, the HA2-based HTM shows higher hole mobility (8.38 × 10−4 cm2 V−1 s−1) and conductivity (1.78 × 10−3 S cm−1) than that of the HA1-based HTM (6.49 × 10−4 cm2 V−1 s−1 and 1.05 × 103 S cm−1), which is ascribed to the introduction of the benzothiadiazole unit expected to enhance the backbone π-coplanarity, resulting in more efficient π–π stacking in HTM films. The results clearly highlight that the two Ag-based HTMs show outstanding conductivity, which can be attributed to the strong face-to-face π–π stacking assisted by Ag⋯Ag forces, that leads to favorable enhancement of conductivity.43 Notably, the charge-carrier mobility and conductivity of HA1 and HA2 are significantly higher than that of the well-known HTM spiro-OMeTAD (5.31 × 10−5 cm2 V−1 s−1 and 8.67 × 10−5 S cm−1),35 indicating that HA1 and HA2 may be suitable candidates as HTMs for PSCs (Fig. 3).
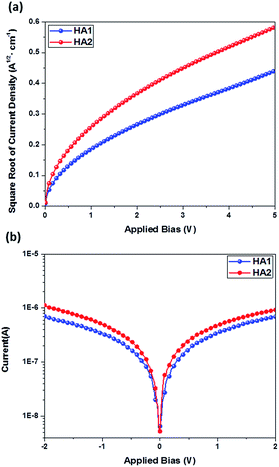 | ||
| Fig. 3 (a) J–V plots of the hole-only devices based on HA1 and HA2. (b) Conductivity characteristics of devices based on HA1 and HA2. | ||
The top-view SEM images of the perovskite film on TiO2 and the HA1 film on perovskite are shown in Fig. S4a and b.† It can be clearly seen that the perovskite film completely covers the TiO2 substrate with rough perovskite nanocrystals, which is similar to that of the perovskite capping layer grown on a mesoporous TiO2 substrate using two-step sequential deposition, after spin-coating a thin HTM HA1 layer on the top of the perovskite film; the surface appears uniform with 100% coverage by the HTM layer. As shown in Fig. S4d,† the thickness of the HA1 layer is estimated to be ∼30 nm, which is thick enough to prevent direct contact between the perovskite layer and the electrode.
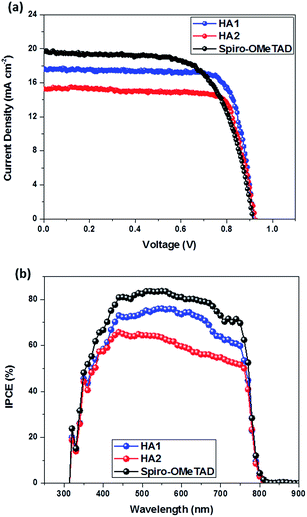
The current–voltage (J–V) curves of these PSCs based on HTMs HA1 and HA2 are shown in Fig. 4 and the corresponding photovoltaic parameters are tabulated in Table 2. The HA1-based PSCs provided a final PCE of 11.98% with a short-circuit current density (Jsc) of 17.28 mA cm−2, an open-circuit voltage (Voc) of 0.912 V and a fill factor (FF) of 0.76, while HA2-based PSCs showed a slightly lower PCE of 10.79% with a Jsc of 15.12 mA cm−2, a Voc of 0.915 V and a FF of 0.78. The lower Jsc value for devices based on HA2 relative to those based on HA1 is in agreement with the incident photon-to-current conversion efficiency (IPCE) spectra with ≈75% maximum conversion for HA1-based cells as compared to ≈60% for HA2-based ones. However, the slightly higher Voc of HA2-based devices should be attributed to the much deeper HOMO energy level, and the higher charge-carrier mobility and conductivity of HA2 are responsible for its higher fill factor. Under the same fabrication conditions, the cell based on spiro-OMeTAD achieved a PCE of 12.27% with a Jsc of 19.57 mA cm−2, Voc of 0.910 V and FF of 0.69. Clearly, the photovoltaic performance of the PSCs employing HA1 as a HTM is comparable to that of the cell based on spiro-OMeTAD. Notably, PSCs based on HA1 and HA2 show a remarkably higher FF than that of spiro-OMeTAD-based devices, which can be attributed to the high metal–organic HTM charge-carrier mobility and conductivity. These promising results indicate that the strategy of designing metal–organic, complex-based HTMs is an attractive route to high-performance PSCs. In order to show the impact of the hysteresis on our device performances, all the devices were prepared and tested under the same working conditions. Notably, PSCs based on HA1 and HA2 showed similar minor hysteretic behavior, while spiro-OMeTAD showed a little bit higher hysteresis behavior, as shown in Fig. S5.† The results indicated that the I–V hysteresis may be influenced by the conductivity of the hole transport material.
| HTMs | J sc (mA cm−2) | V oc (V) | FF | PCE (%) |
|---|---|---|---|---|
| HA1 | 17.28 | 0.912 | 0.76 | 11.98 |
| HA2 | 15.12 | 0.915 | 0.78 | 10.79 |
| Spiro-OMeTAD | 19.57 | 0.910 | 0.69 | 12.27 |
To illustrate the hole extraction and transport of the HTMs, we measured time-resolved photoluminescence (TR-PL) of HTM/CH3NH3PbI3/glass devices, as shown in Fig. S6.† Obviously, the TR-PL in the HTM/CH3NH3PbI3/glass film shows significant decay and much faster than that in the pristine perovskite film, confirming that fast hole transfer from perovskite into the HTM layer.44
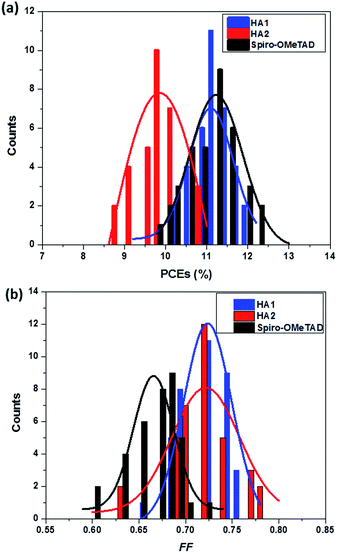
To confirm the reproducibility of device performance, we tested 36 devices that were fabricated using HA1, HA2 and spiro-OMeTAD. Histograms of the cell-performance characteristics are shown in Fig. 5. The average PCEs (Fig. 5a) of the devices with HA1 were comparable to those of the devices with spiro-OMeTAD with high reproducibility. Notably, the average FF of the devices (Fig. 5b) based on HA1 and HA2 was significantly higher than those of the devices with spiro-OMeTAD, indicating that the integration of metal cations into organic anionic materials is propitious to a high fill factor.
Conclusions
In summary, we have designed a new type of hole-transport material based on silver metal–organic complexes as alternatives to the expensive spiro-OMeTAD for PSCs. The recorded electrical conductivity of ∼10−3 S m−1 for this type of HTM in the pristine form is significantly higher than that of spiro-OMeTAD (∼10−5) by over two orders of magnitude. PSCs based on HA1 displayed a PCE of 11.98% under ambient atmosphere conditions, which is comparable to that of the cell employing the commonly used doped spiro-OMeTAD (12.27%), while the HA2-based cell showed a slightly lower performance with a PCE of 10.79%. Moreover, the devices based on the two new HTMs exhibit an extraordinary fill factor of 0.76 (HA1) and 0.78 (HA2). Compared with the state-of-the-art HTM spiro-OMeTAD, these dopant-free HTMs exhibit some beneficial properties, such as the low-cost, easy synthesis and purification with high yields as well as a competitive photovoltaic performance. Our work provides some insightful information for the further development of low-cost HTMs based on metal–organic complexes for future PSC applications.Acknowledgements
This work was financially supported by the Swedish Research Council, the Swedish Energy Agency, the Knut and Alice Wallenberg Foundation, the National Natural Science Foundation of China (21120102036, 91233201), the National Basic Research Program of China (973 program, 2014CB239402). We also greatly thank Dr Lele Duan (KTH) for his helpful discussions.Notes and references
- J. H. Im, C. R. Lee, J. W. Lee, S. W. Park and N. G. Park, Nanoscale, 2011, 3, 4088 RSC.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316 CrossRef CAS PubMed.
- D. Liu and T. L. Kelly, Nat. Photonics, 2013, 8, 133 CrossRef.
- H. P. Zhou, Q. Chen, G. Li, S. Luo, T. B. Song, H. S. Duan, Z. R. Hong, J. B. You, Y. S. Liu and Y. Yang, Science, 2014, 345, 542 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, W. S. Yang, Y. C. Kim, S. C. Ryu, J. Seo and S. I. I. Seok, Nature, 2015, 517, 476 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- H. S. Kim, C. R. Lee, J. H. Im, K. B. Lee, T. Moehl, A. Marchioro, S. J. Moon, R. Humphry-Baker, J. H. Yum, J. E. Moser, M. Grätzel and N. G. Park, Sci. Rep., 2012, 2, 591–597 Search PubMed.
- H. S. Jung and N. G. Park, Small, 2015, 11, 10 CrossRef CAS PubMed.
- T. C. Sum and N. Mathews, Energy Environ. Sci., 2014, 7, 2518 CAS.
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812 CrossRef CAS PubMed.
- N. G. Park, J. Phys. Chem. Lett., 2013, 4, 2423 CrossRef CAS.
- Z. Yu and L. Sun, Adv. Energy Mater., 2015, 1500213 Search PubMed.
- Y. Z. Wu, A. Islam, X. D. Yang, C. J. Qin, J. Liu, K. Zhang, W. Q. Peng and L. Y. Han, Energy Environ. Sci., 2014, 7, 2934 CAS.
- G. E. Eperon, V. M. Burlakov, P. Docampo, A. Goriely and H. J. Snaith, Adv. Funct. Mater., 2014, 24, 151 CrossRef CAS.
- J. A. Christians, R. C. M. Fung and P. V. Kamat, J. Am. Chem. Soc., 2014, 136, 758 CrossRef CAS PubMed.
- P. Qin, S. Tanaka, S. Ito, N. Tetreault, K. Manabe, H. Nishino, M. K. Nazeeruddin and M. Grätzel, Nat. Commun., 2014, 5, 3834 CAS.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087 CrossRef CAS PubMed.
- H. Li, K. Fu, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, Angew. Chem., Int. Ed., 2014, 53, 4085 CrossRef CAS PubMed.
- T. Krishnamoorthy, F. Kunwu, P. P. Boix, H. Li, T. M. Koh, W. L. Leong, S. Powar, A. Grimsdale, M. Grätzel, N. Mathews and S. G. Mhaisalkar, J. Mater. Chem. A, 2014, 2, 6305 CAS.
- H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Chem.–Eur. J., 2014, 20, 10894 CrossRef CAS PubMed.
- Y. Song, S. Lv, X. Liu, X. Li, S. Wang, H. Wei, D. Li, Y. Xiao and Q. Meng, Chem. Commun., 2014, 50, 15239 RSC.
- B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629 CrossRef CAS PubMed.
- S. D. Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. Hong, H. K. Kim and W. I. Lee, Chem. Commun., 2014, 50, 14161 RSC.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963 CAS.
- S. Kazim, F. J. Ramos, P. Gao, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Energy Environ. Sci., 2015, 8, 1816 CAS.
- P. Qin, H. Kast, M. K. Nazeeruddin, S. M. Zakeeruddin, A. Mishra, P. Bäuerle and M. Grätzel, Energy Environ. Sci., 2014, 7, 2981 CAS.
- Y. S. Liu, Q. Chen, H. S. Duan, H. P. Zhou, Y. Yang, H. J. Chen, S. Luo, T. B. Song, L. T. Dou, Z. R. Hong and Y. Yang, J. Mater. Chem. A, 2015, 3, 11940 CAS.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C. S. Lim, J. A. Chang, Y. H. Lee, H. J. Kim, A. Sarkar, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, Nat. Photonics, 2013, 7, 486 CrossRef CAS.
- S. Ryu, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Yang, J. W. Seo and S. I. Seok, Energy Environ. Sci., 2014, 7, 2614 CAS.
- Z. Zhu, Y. Bai, H. K. H. Lee, C. Mu, T. Zhang, L. Zhang, J. Wang, H. Yan, S. K. So and S. Yang, Adv. Funct. Mater., 2014, 24, 7357 CrossRef CAS.
- Y. S. Kwon, J. Lim, H. J. Yun, Y. H. Kim and T. Park, Energy Environ. Sci., 2014, 7, 1454 CAS.
- B. Xu, J. Huang, H. Ågren, L. Kloo, A. Hagfeldt and L. Sun, ChemSusChem, 2014, 7, 3252 CrossRef CAS PubMed.
- J. Burschka, A. Dualeh, F. Kessler, E. Baranoff, N. L. Cevey-Ha, C. Yi, M. K. Nazeeruddin and M. Grätzel, J. Am. Chem. Soc., 2011, 133, 18042 CrossRef CAS PubMed.
- B. Xu, H. Tian, D. Bi, E. Gabrielsson, E. M. J. Johansson, G. Boschloo, A. Hagfeldt and L. Sun, J. Mater. Chem. A, 2013, 1, 14467–14470 CAS.
- P. Liu, B. Xu, K. M. Karlsson, J. B. Zhang, N. Vlachopoulos, G. Boschloo, L. Sun and L. Kloo, J. Mater. Chem. A, 2015, 3, 4420–4427 CAS.
- M. Cheng, C. Chen, X. C. Yang, J. Huang, F. G. Zhang, B. Xu and L. Sun, Chem. Mater., 2015, 27, 1808 CrossRef CAS.
- Y. Song, S. T. Lv, X. C. Liu, X. G. Li, S. R. Wang, H. Y. Wei, D. M. Li, Y. Xiao and Q. B. Meng, Chem. Commun., 2014, 50, 15239 RSC.
- M. Cheng, B. Xu, C. Chen, X. C. Yang, F. G. Zhang, Q. Tan, Y. Hua, L. Kloo and L. Sun, Adv. Energy Mater., 2015, 5, 1401720 Search PubMed.
- A. Krishna, D. Sabba, H. R. Li, J. Yin, P. P. Boix, C. Soci, S. G. Mhaisalkar and A. C. Grimsdale, Chem. Sci., 2014, 5, 2702 RSC.
- H. J. Snaith and M. Grätzel, Appl. Phys. Lett., 2006, 89, 262114 CrossRef.
- T. Leijtens, I. K. Ding, T. Giovenzana, J. T. Bloking, M. D. McGehee and A. Sellinger, ACS Nano, 2012, 6, 1455 CrossRef CAS PubMed.
- K. M. Hutchins, T. P. Rupasinghe, L. R. Ditzler, D. C. Swenson, J. R. G. Sander, J. Baltrusaitis, A. V. Tivanski and L. R. MacGillivray, J. Am. Chem. Soc., 2014, 136, 6778 CrossRef CAS PubMed.
- M. S. Kang, S. D. Sung, I. T. Choi, H. Kim, M. Hong and J. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 22213 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc03569d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |