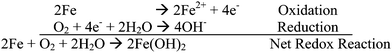Prepare, Do, Review: a model used to reduce the negative feelings towards laboratory classes in an introductory chemistry undergraduate unit
Dino
Spagnoli
 *a,
Lawrence
Wong
b,
Shannan
Maisey
a and
Tristan D.
Clemons
*a
*a,
Lawrence
Wong
b,
Shannan
Maisey
a and
Tristan D.
Clemons
*a
aSchool of Chemistry and Biochemistry, The University of Western Australia, Perth, Western Australia, Australia. E-mail: dino.spagnoli@uwa.edu.au; tristan.clemons@uwa.edu.au; Fax: +61 8 6488 1005; Tel: +61 8 6488 8681 Tel: +61 8 6488 4422
bSchool of Medicine and Pharmacology, The University of Western Australia, Perth, Western Australia, Australia
First published on 20th September 2016
Abstract
Student feelings towards the laboratory component of an introductory chemistry unit were evaluated in an action research study, over a three-year period at the University of Western Australia. In 2013 we found that the percentage of students with negative feelings towards the laboratory increased over the duration of a semester. In 2014 we developed and introduced the use of pre-laboratory online activities, which the students found to be helpful in preparing them for the laboratory. However, there was no change in trend of negative feelings towards laboratory classes from 2013 to 2014. In 2015 we introduced the Prepare, Do, Review model and found that there was a reduction in the percentage of students with negative feelings towards laboratory classes compared with previous years. The Prepare, Do, Review model allows students more time to process the information given in the laboratory. We believe that this model could apply to laboratory programs in any discipline.
Introduction
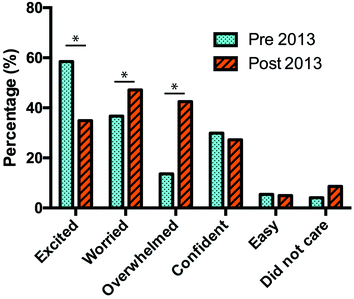
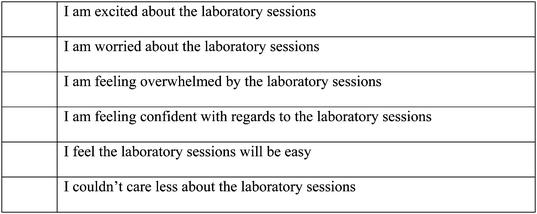
Introductory science units are common in many universities around the world. Due to the importance of chemistry in fields such as the life sciences, earth sciences and engineering, introductory chemistry units are offered in almost all university chemistry departments. Generally, research has focused on the effectiveness of both semester-long (Jones and Gellene, 2005), short, intensive introductory chemistry courses (Schmid et al., 2012), as well as of laboratory experiences (Hofstein and Lunetta, 1982; Hofstein and Lunetta, 2004; Buntine et al., 2007; Reid and Shah, 2007; Elliott et al., 2008). However, we believe that an instrument to assess outcomes from the laboratory experience should evaluate students' feelings, as well as knowledge and skills acquisition (Galloway et al., 2016). Research into students' feelings towards the laboratory has been limited and has focused on the sources of anxiety (Bowen, 1999; Kurbanoglu, 2014) and potential methods of decreasing anxiety (Abendroth and Friedman, 1983). The method developed by Abendroth and Friedman involved the use of a counsellor to develop an anxiety reduction program specific to chemistry.In this study we focus on the laboratory component of an introductory chemistry unit offered to first-year students at the University of Western Australia (UWA). Over a three-year period (2013–2015), with three different cohorts, we asked students to complete a survey at the start of semester (pre-survey) and at the end of semester (post-survey). The surveys asked students to assess their feelings towards the laboratory (excited, worried, overwhelmed, confident, easy, did not care) and we compared the results from the pre-survey with those from the post-survey. The feeling descriptors were chosen on anecdotal evidence as a result of focus group discussions between experienced lecturers and laboratory demonstrators of the unit. In the 2013 pilot study, we found that at the end of semester there was a statistically significant increase in the number of students who indicated negative feelings (worried or overwhelmed) towards the laboratory classes (Fig. 1). The percentage of students that were excited in the laboratories significantly decreased. There was also a decrease in confidence from the start of semester to the end of semester, which although not statistically significant, is still a concern. This change went against our initial expectation. We hypothesised that with increased familiarity of the laboratory and university environment, confidence in the laboratory would increase and negative feelings would decrease. In subsequent years, we introduced two teaching strategies to attempt to reverse the increase in negative feelings towards the laboratory classes.
The first teaching strategy (2014) was the introduction of pre-laboratory online quizzes, videos and reading material. Online videos have been used to replace in-class lectures as a form of “classroom flip” over the last 15 years (Foertsch et al., 2002; O'Flaherty and Phillips, 2015). Previous studies have shown that the use of online quizzes, which provide instant feedback, improves student preparedness for the laboratory activity compared to just reading the laboratory manual (Chittleborough et al., 2007). Providing students with a video, reading material and quizzes before attempting the laboratory has been shown to improve learning outcomes in chemistry laboratory classes (Teo et al., 2014). We investigated if there was a correlation between an increase of student preparation through online pre-laboratory activities and reduction of students' negative feelings toward laboratory classes.
A great deal of new, symbolic and technical information can be introduced to students in a laboratory session (Taber, 2009). We wanted to investigate if there was any correlation between the time students needed to process information and the negative feelings towards the laboratory classes. In 2015, we introduced a second teaching strategy, which was the Prepare, Do, Review (PDR) model. This model, which has been developed for second year biochemistry units at UWA (Arthur et al., 2016) allowed more time for students to process information. We increased the length of time by splitting the laboratory into a two-hour practical session where students would “do” the experiment and then the week after the students would “review” the practical session. We maintained the “prepare” part by implementing the change introduced in 2014.
In this paper we will outline the introductory chemistry unit offered at UWA and describe the research methods. The research instruments used in this paper aim to answer the following research questions:
(1) Is there a relationship between increasing student preparation and reducing students' negative feelings towards the laboratory?
(2) Is there a relationship between increasing the length of time of processing information and reducing the negative feelings students have towards the chemistry laboratory?
Context of the study
The introductory chemistry unit at UWA is a unit delivered in the School of Chemistry and Biochemistry. Students cannot enroll if they have passed a chemistry subject in the final year of high school. Students use the introductory chemistry unit as a means to continue studying in disciplines such as biomedical sciences, biochemistry, genetics, engineering science, and pathology and laboratory medicine. The assessment mechanism of this unit includes an end of semester exam (50%), online quizzes throughout semester (25%) and six laboratory experiments (25%). The average unit grades for students in years 2013, 2014 and 2015 were 65% ± 15, 61% ± 14, and 65% ± 15, respectively. Therefore, we are confident that the academic ability of students between cohorts is very similar. The topics of the laboratory experiments are listed below:• Identification of common ions
• Intermolecular forces
• Chemical equilibrium
• Oxidation and reduction
• Acids and bases
• Molecular models
The theory related to the laboratory classes had been presented in the lectures before students attended the laboratory. The information required to complete the experiment was given in the form of a laboratory manual that the student received at the start of semester. All pre-laboratory information, laboratory procedure and safety information was provided in the laboratory manual. The assessment mechanism was in the form of “fill in the box” style questions. For each laboratory session, students would answer questions based on their observations and chemical knowledge to gain marks for the assessment. The first part of each experimental section contained an introduction for the laboratory, where there were also questions based on the background material covered in the pre-laboratory activity. All other questions were answered in the three-hour laboratory session and submitted at the end of the laboratory for marking.
The research study comprised written surveys, which were conducted at the beginning of the laboratory component of the unit (pre-survey) and again at the end (post-survey). In order to maximise student participation it was decided to collect data using pen and paper. A large percentage of the cohort responded to the surveys. We can be confident that we have a good sample size to make conclusions based on the results of the surveys (Table 1).
| Year | Number of students enrolled | Number of students completing the unit | Number of completed pre-surveys (% based on number of enrolments) | Number of completed post-surveys (% based on number of completions) |
|---|---|---|---|---|
| 2013 | 361 | 340 | 199 (55) | 302 (89) |
| 2014 | 343 | 318 | 166 (48) | 170 (53) |
| 2015 | 301 | 277 | 274 (91) | 224 (80) |
In the surveys, students were given the opportunity to freely express their opinions on the laboratory component, which will be discussed in the Results section of this paper. The survey questions discussed in this paper from all years can be found in Appendix 1. Participation in the study was voluntary and the potential risks and benefits associated with the study were described in participation information sheets. All students signed a consent form at the start of the study and could leave the study at any time (Appendix 2). The Human Ethics Office at UWA reviewed questionnaires, consent form and participation information sheets and this study was authorized to proceed under project number RA/4/1/5939 for a five-year period.
Results and discussion
Introduction of pre-laboratory videos and quizzes-2014
The first change to the laboratory structure was to develop a set of pre-laboratory videos and online quizzes, which were both available from the Learning Management System (LMS). We developed a pre-laboratory video for five of the six laboratories. The acids and bases laboratory did not have a pre-laboratory video due to time constraints. However, all laboratory activities had an online quiz, based on the information in the video (where applicable) and the laboratory manual. Students had the opportunity to do the quiz twice, however, the second attempt involved different questions of the same theme than those used in the first attempt. The highest score of both attempts was used for the students' marks and each quiz was worth 1 mark out of 10 for the laboratory. Students received feedback on their answers after each attempt. All other aspects of the laboratory (duration, position in the semester and content) were consistent between 2013 and 2014. The videos were hosted on the YouTube website. This allowed for the analysis of the data provided in YouTube to evaluate if students were watching the videos and for how long (Table 2). The pre-laboratory videos were posted on YouTube as unlisted videos. This means that the videos do not show up on any searches made by the general public on YouTube or other search engines. Therefore, we can be confident that only students that had access to the LMS unit could have had access to the direct YouTube link and, in turn, the prepared videos.| Video (length of video (min:sec)) | Number of views in semester 1 2014 (number of students enrolled in the unit = 343) | Average view duration (min:sec) |
|---|---|---|
| Identification of common ions (7:11) | 584 | 4:52 |
| Intermolecular forces (3:34) | 505 | 2:47 |
| Chemical equilibrium (10:08) | 456 | 5:43 |
| Oxidation and reduction (8:22) | 438 | 6:06 |
| Molecular models (9:44) | 419 | 6:59 |
The analytics obtained from YouTube (Table 2) confirm that there were more student views than the number of students enrolled in the unit and that students' watched on average more than half of the video. The lowest average view duration was 56% of the chemical equilibrium video and the highest average duration was 78% for the intermolecular forces video. The number of views and average view duration of each video implies that some students were watching the videos multiple times. However, it is likely not in entirety on repeat views, hence the reduction in view duration. There was a spike in viewing for all videos the day before the laboratory session as the deadline for the online quiz was set to midnight on the evening before the laboratory. This suggests that students were watching the videos before answering the questions in the online quiz.
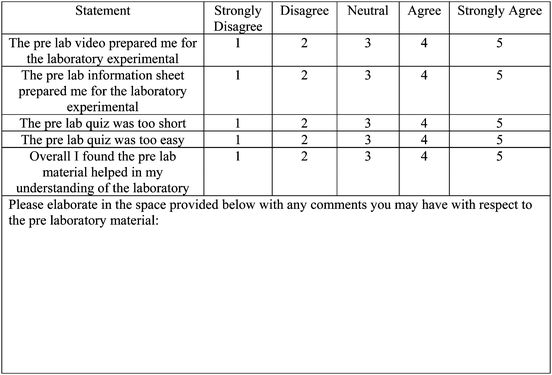
Pre- and post-laboratory course surveys were used again in 2014. We wanted to establish if the pre-laboratory videos and quizzes prepared students for the laboratory. In the post-survey, students were asked to respond to a five-item Likert scale on the following statements:
• The pre-laboratory video prepared me for the laboratory experimental
• The pre-laboratory information in the lab manual prepared me for the laboratory experimental
• The pre-laboratory quiz was too short
• The pre-laboratory quiz was too easy
• Overall, I found the pre-laboratory material helped in my understanding of the laboratory
Overall, students found the online material useful in preparation for the laboratory practical, with the percentage of agree or strongly agree at 70.3% for online video and 67.1% for information from the laboratory manual (Table 3). The majority of students did not think that the online quiz was too short or too easy. Students indicated that the pre-laboratory material helped in their understanding of the laboratory with a percentage of agree or strongly agree recorded at 77.0%. This was encouraging that students were using the pre-laboratory material to prepare for the laboratory and found it useful. Our results are in line with a number of other studies that evaluated the effect of pre-laboratory material delivered online (Chittleborough et al., 2007; Teo et al., 2014).
| Statement | % Strongly disagree | % Disagree | % Neutral | % Agree | % Strongly agree |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| The pre-lab video prepared me for the laboratory experimental | 1.3 | 5.3 | 23.0 | 53.9 | 16.4 |
| The pre-lab information in the lab manual prepared me for the laboratory experimental | 2.0 | 5.9 | 25.0 | 52.0 | 15.1 |
| The pre-lab quiz was too short | 11.8 | 33.6 | 31.6 | 17.1 | 5.9 |
| The pre-lab quiz was too easy | 7.9 | 28.3 | 40.1 | 16.4 | 7.2 |
| Overall, I found the pre-lab material helped in my understanding of the laboratory | 1.3 | 5.9 | 15.8 | 57.9 | 19.1 |
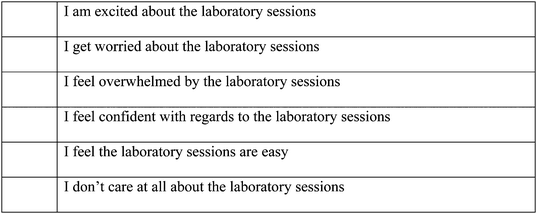
In the 2014 survey we asked students about their feelings with regards to the laboratories in the pre- and post-survey. We can compare the responses collected in 2014 with the responses collected in 2013 (Table 4).
| Feelings | Traditional laboratory format | Introduction of pre-lab online activities |
|---|---|---|
| Difference between pre and post-survey responses 2013 | Difference between pre and post-survey responses 2014 | |
| Excited | Decrease by 23.6% | Decrease by 18.8% |
| Worried | Increase by 10.4% | Increase by 8.8% |
| Overwhelmed | Increase by 28.9% | Increase by 12.7% |
| Confident | Decrease by 2.7% | Increase by 2.6% |
| Easy | Decrease by 0.5% | Increase by 3.8% |
| Did not care | Increase by 4.6% | Increase by 4.5% |
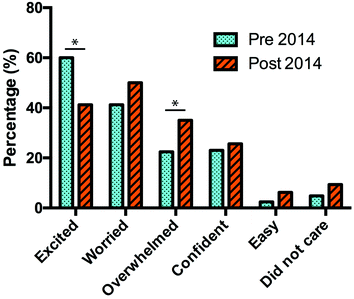
In 2014, the increase in percentage of students that were worried at the end of semester compared to the start of semester increases, but not to a level that was statistically significant. The percentage of students that were confident at the start of semester was at a higher level compared to the end of semester, which is an improvement, compared with the 2013 results, but not to a significant level. However, even though a lower percentage of students felt overwhelmed by the laboratories in 2014 compared with 2013, there is still a significant increase from start to end of semester. Moreover, the general decrease in the level of excitement towards the laboratory was the same in 2013 as in 2014 and is still a significant change (Fig. 2 and Table 4).
We asked students to freely provide their opinions on the pre-laboratory material. Comments varied from positive comments such as, “Pre lab material is great” and “I thought the pre labs were easy, but I thought the labs were easy too so that isn't saying much. The videos were certainly very helpful” to negative comments such as, “They didn't really prepare for the difficulty of questions (in the laboratory)” and “The actual lab is a lot more complicated than what it seems in the pre lab”. The quantitative data we obtained in the pre- and post-surveys from 2014 indicated that the pre-laboratory material helped prepare the students for the laboratory but the levels of negative feelings towards the laboratory component of the unit did not change compared to the traditional laboratory approach.
Introduction of the Prepare, Do, Review Model—2015
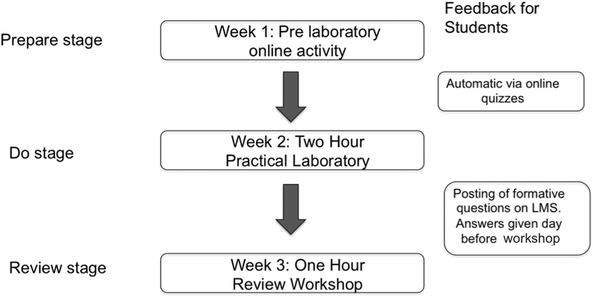
In 2015 the strategy that we employed was to spread the information, content and assessment of the laboratory over a three-week period. The flow diagram below describes the structure of the PDR model (Fig. 3).The “Prepare” stage of the PDR model had already been adopted in 2014, where it consisted of an online video, online reading and an accompanying quiz to assess the students and provide feedback on their answers. The preparation activity was open to students the week before the practical laboratory. The “Do” stage of the PDR model was to isolate the practical component of the laboratory into a two-hour session. Between the “Do” stage and the “Review” stage, a set of formative questions were posted on the LMS to prepare the students for the upcoming review workshop. The hour-long workshop was held in the third week and made up the Review stage of the PDR model. There are two important aspects of this model in relation to the previous three-hour laboratory session that was used in 2013 and 2014. Firstly, the worksheet questions were separated out into practical based observations and calculations for the “Do” stage, and understanding and problem solving for the “Review” stage. Therefore, the assessment questions were consistent from 2013 to 2015. In the workshops, students work collaboratively to answer the questions in the worksheets. The students are encouraged by demonstrators and staff to reflect on their experiences in the practical component, which develops critical thinking and problem solving skills. An example of the worksheets for the “Do” and “Review” workshops and the formative questions can be found in Appendix 3 and 4 respectively. Secondly, this model incurred no additional cost to the School of Chemistry and Biochemistry because the practical laboratories were not changed and the face-to-face time with the sessional staff (laboratory demonstrators) remained constant. The only difference was that the three-hour face-to-face time was spread over two weeks. This model required students to think about the laboratory over a three-week period, rather than three hours in one week, which was common in the 2013 laboratory format. The distribution of marks for the PDR model was as follows:
• Prepare stage (online pre-laboratory quiz) – 1 mark out of 10
• Do stage (laboratory class) – 6 marks out of 10
• Review stage (1 h workshop) – 3 marks out of 10
Five out of the six laboratories adopted the PDR model. The only exception was the molecular models laboratory as the nature of that laboratory did not fit into the PDR model. There was no explicit experimental component to the molecular models laboratory. There may be scope to develop this laboratory for the PDR model in future years.
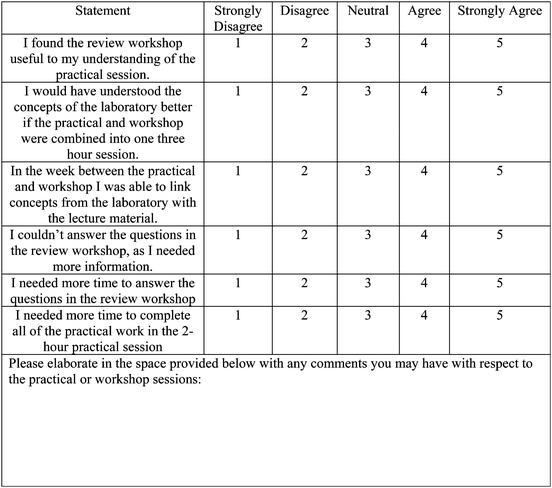
The same questions asked in the 2013 and 2014 surveys were included in pre- and post-surveys in 2015. This included the question on the pre-laboratory material (Table 3) and the results and trends were very similar in 2015 compared to 2014. The 2015 post-survey also asked students to respond to a five-item Likert scale on the following statements about the practical and workshop format:
• I found the review workshop useful to my understanding of the practical session
• I would have understood the concepts of the laboratory better if the practical and workshop were combined into one three hour session
• In the week between the practical and workshop I was able to link concepts from the laboratory with the lecture material
• I couldn't answer the questions in the review workshop, as I needed more information
• I needed more time to answer the questions in the review workshop
• I needed more time to complete all of the practical work in the 2 hour practical session
The majority of students agreed or strongly agreed (83.5%) that the review workshop was useful to their understanding of the practical session. Nearly half of the students (48.6%) disagreed or strongly disagreed that they would have understood the concepts of the laboratory better in one 3 hour session. This model enabled a large majority of students (63.4%) who agreed or strongly agreed to link the concepts of the laboratory to the lecture material (Table 5). The links between lecture and laboratories is also supported by previous research, which is important in understanding chemical concepts (Cooper and Foy, 1967).
| Statement | % Strongly disagree | % Disagree | % Neutral | % Agree | % Strongly agree |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| I found the review workshop useful to my understanding of the practical session. | 0.9 | 1.8 | 12.1 | 62.5 | 21.0 |
| I would have understood the concepts of the laboratory better if the practical and workshop were combined into one three hour session. | 14.7 | 33.9 | 26.8 | 18.3 | 3.6 |
| In the week between the practical and workshop I was able to link concepts from the laboratory with the lecture material. | 0.4 | 5.8 | 26.8 | 50.0 | 13.4 |
| I couldn't answer the questions in the review workshop, as I needed more information. | 10.7 | 31.3 | 31.7 | 20.1 | 3.6 |
| I needed more time to answer the questions in the review workshop | 5.4 | 35.7 | 24.1 | 25.0 | 6.3 |
| I needed more time to complete all of the practical work in the 2 hour practical session | 5.8 | 35.7 | 20.1 | 25.4 | 10.3 |
In the post-survey, students were given the opportunity to express their opinions on the practical and/or workshop sessions, and positive and negative examples are detailed in Table 6. The students' comments were insightful and, although there were some negative comments, the majority of students gave positive feedback towards the adoption of the new model. The positive comments shed light on one of the research questions. Namely, students found that they had more time to consolidate the information. Moreover, the negative comments are more aligned to the logistics of students going onto campus for only one hour and the time constraints of the session. Two additional questions were also asked in the post-survey with regards to the formative questions that were posted on the LMS. The formative questions were described as a way of preparing students for the assessments in the review workshop. The formative questions were not assessed or graded in any way and students had access to the formative questions the day after the practical session. Students received the answers to the formative questions the day before the review workshop. We wanted to know if students read and attempted the formative questions and, if they had, whether they found them useful to prepare for the review workshop. Half of the students stated that they had read and attempted the formative questions and of those students 89% found them useful to prepare for the review workshop. This high percentage of students that attempted the non-assessed questions is encouraging as it indicates that the majority of students were thinking about the laboratory in the week between the practical and the review session. This was one of the aims of the PDR model.
| Positive comments |
| • Separate them and have more practical and more workshops instead of a third lecture. I found I learned much more and it sunk in better in the workshops and labs. |
| • I liked the structure of the labs one week, workshop the second week. It gave me more time to consolidate the concepts and apply the knowledge from the lectures/readings. My marks in the reviews improved dramatically over the course of the semester as I gained a better understanding of the material. |
| • I think it was good that the labs were divided because 3 hours would have been too long + I would have stopped concentrating. |
| • I think it is a good method, 2 hour lab one week, 1 hour review the next week. Allows time for me to better understand the concepts of the lab in preparation for the review. |
| • As I took the unit last year, I preferred the labs being split into a prac. and review. |
| • Didn't like it at first, but the system grew on me. 3 hours in one session would feel rushed. |
| Negative comments |
| • I felt that the practical and workshop sessions were quite pressured and confusing. Sometimes a few more demonstrations/explanations would be helpful. I felt I came reasonably well prepared to the sessions but felt a bit lost in the actual sessions. |
| • Splitting the workshop and lab and having 2 sessions meaning there is an assessed session EVERY week made things difficult and annoying. Some weeks I'd have to travel for 30 minutes each way in to uni. just for a review workshop that lasted less than 1 hour. |
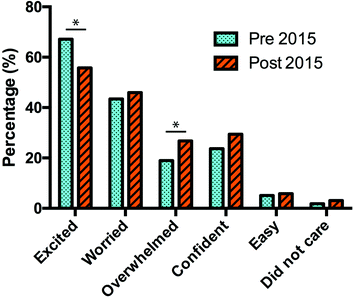
Based on the 2015 pre- and post-surveys we find that there is still an increase in negative feelings (worried or overwhelmed) over the duration of the semester, but the difference from the start to end of semester is far less than in previous years. The increase in the percentage of worried students is not a significant change, which is a similar result found in 2014. Moreover, with the introduction of the PDR model there has been an increase in confidence towards the laboratory compared with previous years (Fig. 4) albeit a non-significant change.
Discussion and statistical analysis
We can compare and contrast the student's feelings towards the laboratory component over the three years of the unit and the changes in trends based on the introductions of new formats (Table 7). We have highlighted the differences in feelings from the start of semester (pre) to the end of semester (post) by looking at the difference between the two percentages. There is a commonality with the results presented in Table 7 between 2014 and 2015. The introduction of the pre-laboratory online activities had a positive impact on confidence and sense of ease when comparing the pre- and post-surveys from 2014 to 2013. The introduction of the PDR model had the same effect, however, the difference in negativity towards the laboratory is now far less in 2015 than using the traditional laboratory format in 2013. Based on these results we conclude that overall the PDR model had a positive impact on students' feelings towards the laboratory component of the unit across the semester compared to the traditional format of laboratories.| Feelings | Traditional laboratory format | Introduction of pre-lab online activities | Introduction of PDR model |
|---|---|---|---|
| Difference between pre and post-survey responses 2013 | Difference between pre and post-survey responses 2014 | Difference between pre and post-survey responses 2015 | |
| Excited | Decrease by 23.6% | Decrease by 18.8% | Decrease by 11.4% |
| Worried | Increase by 10.4% | Increase by 8.8% | Increase by 2.6% |
| Overwhelmed | Increase by 28.9% | Increase by 12.7% | Increase by 7.8% |
| Confident | Decrease by 2.7% | Increase by 2.6% | Increase by 5.8% |
| Easy | Decrease by 0.5% | Increase by 3.8% | Increase by 0.7% |
| Did not care | Increase by 4.6% | Increase by 4.5% | Increase by 1.3% |
A Chi-square test of independence was performed to compare the feelings of students towards the laboratory at the start to the end of semester (Table 8). The difference between students who indicated excitement at the start of semester was significantly lower compared with excitement levels at the end of the semester in all years. However, the p-value increases each year from 2013 through to 2015. This trend is very similar to the number of students that feel overwhelmed by the laboratories. There is a significant increase in the number of students that feel overwhelmed by the laboratories in 2013–2015 from the start to the end of semester. However, the p-value increases each year from 2013 through to 2015. The results of the Chi-square test of independence (Table 8) and the comparison of change in trends (Table 7) both indicate that the introduction of pre-laboratory videos and the PDR model is reducing the amount of negative feelings towards the laboratory. Moreover, the Chi-square test of independence for the increase in the number of students worried about the laboratories is significant in 2013 but not significant in 2014 and 2015. This suggests the interventions introduced in 2014 and 2015 had a significant improvement on students' worry towards the laboratories.
| Feelings | 2013-traditional laboratory format | |||
|---|---|---|---|---|
| Chi square | p-value | Significant | Interpretation | |
| Excited | 22.5291 | 0.0000 | Yes | Association between unit and feeling (decrease) |
| Worried | 4.3754 | 0.0365 | Yes | Association between unit and feeling (increase) |
| Overwhelmed | 37.3387 | 0.0000 | Yes | Association between unit and feeling (increase) |
| Confident | 0.3534 | 0.5522 | No | No association between unit and feeling |
| Easy | 0.0427 | 0.8363 | No | No association between unit and feeling |
| Did not care | 3.0912 | 0.0787 | No | No association between unit and feeling |
| Feelings | 2014-introduction of pre-lab online activities | |||
|---|---|---|---|---|
| Chi square | p-value | Significant | Interpretation | |
| Excited | 11.4258 | 0.0007 | Yes | Association between unit and feeling (decrease) |
| Worried | 2.5294 | 0.1117 | No | No association between unit and feeling |
| Overwhelmed | 6.289 | 0.0122 | Yes | Association between unit and feeling (increase) |
| Confident | 0.2972 | 0.5856 | No | No association between unit and feeling |
| Easy | 2.8843 | 0.0894 | No | No association between unit and feeling |
| Did not care | 2.5309 | 0.1116 | No | No association between unit and feeling |
| Feelings | 2015-introduction of PDR model | |||
|---|---|---|---|---|
| Chi square | p-value | Significant | Interpretation | |
| Excited | 6.7418 | 0.0094 | Yes | Association between unit and feeling (decrease) |
| Worried | 0.3248 | 0.5688 | No | No association between unit and feeling |
| Overwhelmed | 4.3098 | 0.0379 | Yes | Association between unit and feeling (increase) |
| Confident | 2.0959 | 0.1477 | No | No association between unit and feeling |
| Easy | 0.1158 | 0.7336 | No | No association between unit and feeling |
| Did not care | 0.886 | 0.3466 | No | No association between unit and feeling |
Conclusion
Over a three-year period we have surveyed students to evaluate their feeling towards the laboratory component of a unit. Based on the findings of the 2013 questionnaires, we developed different teaching strategies with the aim of reducing the level of negative feelings towards the laboratories that accumulated across the semester. The introduction of pre-laboratory online activities, which were introduced in 2014, did aid in the preparation of students for the laboratory. The percentage of students that were confident about the laboratories before the semester started were at a similar level at the end of the semester, which was in contrast to the decline in the 2013 study. The introduction of the PDR model, which was introduced in 2015, increased students' confidence towards laboratories and reduced negative feelings of being overwhelmed and worried compared with the results in 2013. This study reveals that changing the format of the laboratory can have a significant effect on the number of students worried about the laboratory. This study also highlights the importance of measuring student's feelings towards the laboratory. Further studies in this area should include use of surveys (Bowen, 1999) to discover the source of the negative feelings towards the laboratory.Overall, the study has provided insight into the development of different teaching strategies that could be used to reduce the negative feelings towards the laboratory component of a unit. The PDR format could be used in other introductory science units at a tertiary level where the amount of new information presented to students could be overwhelming and require more time to process.
Appendix 1
2013–2015 pre-survey question
How do you feel about the compulsory laboratory sessions required to be completed for this unit?(Place a tick next to all statements you feel apply to you)
2013–2015 post-survey question
Now that you have completed the majority of the compulsory laboratory sessions for this unit, how do you feel about this component of the unit CHEM 1003? (Place a tick next to all statements you feel apply to you)2014–2015 post survey question on the pre laboratory online activities
Each laboratory contained a pre laboratory section that involved either a pre lab video, which needed to be watched, and/or an information sheet, which needed to be read before your lab. There was also an online quiz for each laboratory. Think about the below statements and please indicate your level of agreement by circling a number from 1 to 5 for every statement.2015 post survey question on the PDR
Each laboratory was split into a practical 2 hour session and a 1 hour review workshop, which occurred the week after. After the 2 hour practical session post laboratory questions (non-assessed) were posted on the LMS website to help prepare you for the 1 hour review workshop. Did you read and attempt those questions (please tick in the boxes below):If you answered YES to the question above, did you find the post laboratory questions useful to prepare you for the review workshop? (Please leave blank if you answered NO to the question above).
Appendix 2
Student Consent and Participant Information SheetAn observational study of student perception of the content and laboratory sessions in CHEM1003
—Consent Form—I ———— (the participant) have read the information provided and any questions I have asked have been answered to my satisfaction. I agree to participate in this activity, realising that I may withdraw at any time without reason and without prejudice.
I understand that all identifiable (attributable) information that I provide is treated as strictly confidential and will not be released by the investigator in any form that may identify me. The only exception to this principle of confidentiality is if documents are required by law.
I have been advised as to what data is being collected, the purpose for collecting the data, and what will be done with the data upon completion of the research.
I agree that research data gathered for the study may be published provided my name or other identifying information is not used.
——————————— ——————————
Participant Date
An observational study of student perception of the content and laboratory sessions in CHEM1003
—Participant Information Sheet—Purpose
This project aims to investigate students' own perceived motivations and reasons behind undertaking the introductory chemistry unit CHEM1003. We plan to assess how students' motivations for studying the unit are affected by previous experiences with chemistry education as well as students future career aspirations. We also wish to get feedback from you on the introduction of online pre laboratory tutorials and assessments.Procedures
Your participation in this study requires you to participate in three (3) surveys, which will be made available to you during your lecture and laboratory classes. These surveys will be spaced out throughout the semester (a total of 3 surveys to complete) and will carry no marks towards the unit. The surveys are short in length to ensure minimum time commitment and disruption to participants is maintained throughout the study.Upon completion of the first survey students will have the opportunity to also provide consent to be involved in an interview process (answer with a tick in question 14 survey 1 to indicate consent). Researchers may approach students whom indicate they are willing to participate in interviews during their compulsory laboratory sessions to provide further anecdotal evidence and examples surrounding their responses to the answers provided in the student survey. These interviews will be recorded and will be transcribed and stored only on a secure hard drive until the study is complete. Once the study is completed all digital audio files and hard copies of associated documents will be permanently erased.
Risks
The researchers acknowledge that a potential risk of this study could be the added time commitment required to be a participant in this study. However every effort will be made to ensure any commitment for the study will be flexible, succinct and completed during course time. Furthermore it is anticipated the surveys will only take 10 minutes maximum and will be done in class time so the external commitment by students is minimised.Benefits
Research team
Dr Dino Spagnoli will be the chief investigator for this study however due to his direct involvement in the teaching and organisation of the laboratory sessions he will not be involved in the collection of surveys and interview questioning to avoid this conflict of interest. For the data collection and conduction of interviews Dr Tristan Clemons (research fellow in the School of Chemistry and Biochemistry) will be the primary researcher involved in this work with the guidance of Prof. Bob Bucat. Both Tristan and Bob alike declare no conflict of interest with students enrolled in the unit CHEM 1003 and will not be directly involved in the teaching and/or assessment of this unit to ensure the integrity of this study is maintained.Confidentiality of data
Personal details and survey responses will be treated confidentially at all times. Individual data will not be identifiable by any staff member at the University of Western Australia whom is directly involved in the teaching and assessment of the unit CHEM1003, but collective results may be published with alias' used for any case study information provided. Data will be stored on a password-protected laptop, saved to an external hard drive, which will be stored in a secure private office of which only research personnel will have access to.Subject rights
Participation in this research is voluntary and you are free to withdraw from the study at any time and for any reason, without prejudice in any way. If you withdraw from the study and you are an employee or student at the University of Western Australia (UWA), this will not prejudice your status and rights at UWA at all. Your participation in this study does not prejudice any right to compensation that you may have under the statute of common law. Further information regarding this study may be obtained from Dr Tristan Clemons (E-mail: tristan.clemons@uwa.edu.au; ) or from Dr Dino Spagnoli (dino.spagnoli@uwa.edu.au). The committee for Human Rights at the University of Western Australia requires that all participants are informed that, if they have any complaint regarding the manner, in which a research project is conducted, it may be given to the researcher or, alternatively to the Secretary, Committee for Human Registrar's Office, University of Western Australia, Crawley, WA 6009 (telephone number 6488 3703). All study participants will be provided with a copy of the information sheet and consent form for their personal records.Thank you for your time and cooperation in this work.
Best regards,
Dr Dino Spagnoli
Approval to conduct this research has been provided by the University of Western Australia, in accordance with its ethics review and approval procedures. Any person considering participation in this research project, or agreeing to participate, may raise any questions or issues with the researchers at any time. In addition, any person not satisfied with the response of researchers may raise ethics issues or concerns, and may make any complaints about this research project by contacting the Human Research Ethics Office at the University of Western Australia. All research participants are entitled to retain a copy of any Participant Information Form and/or Participant Consent Form relating to this research project.
Appendix 3
Example work sheet used for the practical session in the PDR modelExperiment 5: practical-oxidation and reduction
In this experiment you will investigate some electron-transfer reactions, examining the relative electron-attracting strengths of some species and simulating the extraction of gold and the electrochemical processes that take place.Introduction
An electron-transfer reaction involves the transfer of electrons from the reduced member of one redox species to the oxidised member of another redox species.An electron-transfer reaction involves both an oxidation and a reduction in the one process. Oxidation occurs when the electron is lost and reduction occurs when the electron is gained.
Oxidation and reduction MUST occur together. Such a reaction is a REDOX reaction. The arguments are as follows:
• If one species loses electrons, another must gain them.
• If one atom increases its oxidation number, the electrons lost must be counted elsewhere, so that another atom decreases its oxidation number.
When a redox reaction takes place, each half reaction has associated to it, a standard reduction potential (page xxviii of lab manual) that is seen as the tendency to acquire electrons and proceed as a reduction.
When two half cells are connected, the one with the larger reduction potential (the greater tendency to undergo reduction) acquired electrons from the half-cell with the lower reduction potential, which is therefore forced to undergo oxidation.
For a redox reaction to occur spontaneously, the overall cell potential must be positive. The overall cell potential is the difference between the reduction potential of one half-cell and the reduction potential of the other, and overall, for a redox reaction to take place without external interference, this overall potential must be positive.
The standard reduction potentials for the possible half-cells are shown on the table of electrode potentials and list the half equations in order of most positive to least positive (most negative). For any pair of half-reactions, the one with the more positive reduction potential will occur as a reduction. The other half-reaction is reversed and occurs as an oxidation.
Redox equations are a form of electrochemistry, and are the chemical equations that explain the electrochemical processes taking place in a system. This theory is applied in many different industries. Electrochemical processes are used to monitor and prevent corrosion, to extract and refine gold from impure ores, to generate electricity in batteries as well as in biological systems such as photosynthesis (just to name a few!).
Photosynthesis is a complex series of coordinated redox reactions, but the overall effect is to reduce gaseous CO2 to carbohydrates and to oxidise H2O to oxygen. (If x = 6, the carbohydrate is glucose).
| xCO2 + xH2O → (CH2O)x + xO2 |
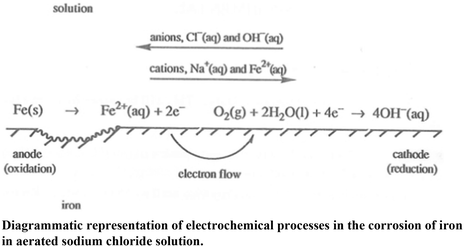
Corrosion is a major problem in society as it causes damage to infrastructure, cars, ships etc that costs ‘tens of billions of dollars’. Monitoring corrosion is very important. It attempts to assess the useful life of equipment and infrastructure and how effective the controls are at preventing major damage to these. Damage can affect production or cause severe environmental harm. Examples of corrosion include the rusting of nails and of old cars for example. This annoying and expensive problem is a result of moisture (H2O) and oxygen (O2) being reduced to hydroxide ions (OH−) when in contact with iron and other metals, and the metal, iron in this case, being oxidised from Fe to Fe2+ or further to Fe3+ ions.
Experimental
Section 1a – corrosion-one metal system
Corrosion of a metal is the result of oxidation of the metal by some substance present in its environment. The most familiar corrosion process is the rusting of iron in air.The mechanism of the first stage in the rusting of iron is similar to that of an electrochemical cell:
Particular reagents can be added to solution in which iron is corroding to show where oxidation and reduction are occurring:
(a) Hexacyanoferrate(III) ions, Fe(CN)63−, reacts with iron(II) ions produced by corrosion to form a deep blue substance called “Prussian Blue”.
(b) Phenolphthalein, an acid–base indicator, turns from colourless to pink in the region where OH− ions are produced.
You will be provided with a warm solution containing small amounts of potassium hexacyanoferrate(III), phenolphthalein, salt and agar. This is called “ferroxyl solution”. As it cools it sets into a jelly. This ferroxyl solution will be used to show:
(a) the preferred regions at which oxidation and reduction occur in particular situations
(b) that certain metals in contact with iron prevent, whereas others hasten, the corrosion of iron.
Note:
• Do NOT place anything into the bulk ferroxyl solution (including spatulas, etc.)
• Do NOT allow the bulk ferroxyl solution to cool to room temperature before use, as it will set.
• Allow 30 minutes for the colour development.
• The ferroxyl indicator does not turn blue in regions where zinc is oxidized. However, a white precipitate of zinc hydroxide, Zn(OH)2, is formed where zinc is oxidized.
Clean one piece of magnesium metal and one piece of zinc metal with emery paper.
Add approximately 20 mL of ferroxyl solution into each of the two 100 mL beakers. After the mixture has gelled, use forceps to insert small pieces of cleaned Zn metal and cleaned Mg metal into each of the beakers with gel. Allow to stand for 30 minutes.
Record your observations of the changes that occur in the Beaker with the magnesium metal. What do you think is occurring to cause any colour changes in the gel?
Record your observations of the changes that occur in the beaker with the zinc metal. What do you think is occurring to cause any colour changes in the gel?
The bulk of a gel is water that has been immobilised by a tangle of agar fibres. This water still contains solute K+, Na+, H+, OH−, Fe2+, Cl− and CN− ions that are all good electrolytes that can travel throughout the gel.
Which of the two metals, Mg or Zn, reacted more spontaneously? How can you explain this? (NB: the reduction potential table should be used to help you determine this).
Section 1b – two metal system
• Clean three iron nails with emery paper.• Wrap a strip of cleaned zinc metal around one nail so that the metals touch. Likewise wrap a strip of cleaned copper metal around the other nail.
• Obtain three 100 mL beakers.
• Pour about 5 mL of ferroxyl solution into each beaker and allow it to set.
• Place the nails on the ferroxyl jelly in separate beakers.
Beaker 1: Steel nail only. This will serve as a comparison for the reactions in the other beakers.
Beaker 2: Steel nail in contact with zinc.
Beaker 3: Steel nail in contact with copper.
• Cover the nails with another 5 mL of ferroxyl solution.
Record your observations of the changes that occur in beaker 1:
Record your observations of the changes that occur in beaker 2:
Record your observations of the changes that occur in beaker 3:
Dispose of the ferroxyl jelly in the rubbish bins provided and rinse all glassware with hot water
Section 2 – extraction of copper
CAUTION: Gloves should be worn when handling the ethylenediamine solution and extra care should be taken. Avoid contact with the skin as allergic reactions may occur.♦ Weigh about 5 g of the “simulated” ore into a 100 mL conical flask.
♦ Add about 20 mL of a 5% aqueous solution of ethylenediamine.
What physical change is observed? What do we now have in solution ?
♦ With continuous swirling, add successive portions of about 0.5 mL of 3% (10 volume) hydrogen peroxide solution. Allow bubbling to subside between additions. Add a total of 20 mL of the hydrogen peroxide solution. Swirl for another 5 minutes.
The bubbling is due to evolution of oxygen produced by decomposition of hydrogen peroxide.
♦ Pour off the solution into a beaker, trying to avoid any of the slurry getting into the beaker as well.
♦ Wash the residue several times with a small volume of water and pour off solution into the beaker.
What is the purpose of washing the residue with water and pouring off again?
Why is hydrogen peroxide added? What is its role?
♦ Add a pea-sized sample of powdered zinc and stir the solution. Wait until the solution is colourless (more zinc may need to be added). Pour off the solution, leaving behind the residue.
Why is zinc added? What is its purpose?
♦ Wash the residue and pour off the liquid at least twice. The black residue is a mixture of copper metal and surplus (unreacted) zinc. Remove the zinc by adding about 4 mL of 3 M sulfuric acid solution.
Record your observations of the changes that occur during section 2:
Experiment 5: workshop-oxidation and reduction reactions
Analysis of section 1a
Q1. The gel that you used in the practical contained good electrolytes such as K+, Na+, H+, OH−, Fe2+, Cl− and CN− ions. What does it mean by a ‘good electrolyte’? Why must the ions be good electrolytes ?Q2. Write both half-equations and the full redox equation for the metals in the gel from the practical in section 1.
Magnesium:
Zinc:
Analysis of section 1b
Q3. Which metal in beaker 2 is higher in the Standard Reduction Potential series found on page xxxii of the laboratory manual ?
Q4. From your observations that you made last week, in beaker 2, when iron and zinc are in contact, which metal is the anode and which metal is the cathode? Explain your answer.
Q5. From your observations that you made last week, compare the colours that formed in beakers 1 and 2. What effect has zinc had on the corrosion of iron?
Q6. Which metal in beaker 3 is higher in the Standard Reduction Potential series found on page xxviii of the laboratory manual?
Q7. From your observations that you made last week, in beaker 3, when iron and copper are in contact, which metal is the anode and which metal is the cathode? Explain your answer.
Q8. From your observations that you made last week, compare the colours that formed in beakers 1 and 3. What effect has copper had on the corrosion of iron?
Q9. Which provides the better long-term protection against the corrosion of steel, a thin layer of copper or a thin layer of zinc? Why?
Critical thinking question
Q10. In WA, aluminium is mined in the form of aluminium oxide (Al2O3). This is commonly known as the bauxite ore. The Bayer process is used to extract and refine pure aluminium. This involves dissolving the bauxite ore to remove the impurities and then precipitating out the crude aluminium oxide. This is then shipped outside of WA to obtain aluminium metal using electrochemical processes. The Al2O3 is in the molten state when this process occurs. Would the Al3+ ions still be reduced to Al metal if it was in an aqueous solution (i.e. if water molecules were present). Explain with the aid of equations and standard reduction potentials.Appendix 4
Example of Post Practical Session Formative question used before the workshopExperiment 5 – oxidation and reduction reactions
You might find it useful to answer the following questions soon after completing your practical lab session;– What do you think were the key areas of Chemistry that were examined in the lab?
– Summarise your finding from the lab in a paragraph.
– What areas of the lab did you find difficult or confusing? (These are areas to focus on in your study).
Use these questions to help you prepare for the post-lab workshop;
(1) What is an electrolyte? How do they work to assist in electrochemical cells?
(2) Write the half equations and then full redox equation for the following cells.
Al/Ni2+
Zn/Pb2+
Ni/Cd2+
(3) We refer to reactions occurring ‘spontaneously’ describe what this means in terms of an electrochemical cell. (hint: think about standard reduction potentials).
(4) Describe how you can use reduction tables to predict which will be the anode and which will be the cathode in a chemical wet cell.
(5) How does the position of a reaction on the reduction potential table influence whether it is likely to occur in the forward or reverse direction? What is the importance of the difference between the two reactions?
(6) Magnesium sacrificial anodes are used on boats to promote the corrosion of an expendable metal block rather than the corrosion of the iron hull. Can you describe, using their relative positions on the standard reduction table, why this is an effective measure to protect the hull of the boat?
Acknowledgements
The authors would like to thank all the students from the Introductory Chemistry unit during years 2013–2015 for agreeing to participate in this study. The Human Research Ethics Office at the University of Western Australia authorized collection of data in this study under project number RA/4/1/5939. The authors would like to thank Karina Price and Bob Bucat for useful discussions around the design of the questionnaires.References
- Abendroth W. and Friedman F., (1983), Anxiety Reduction for Beginning Chemistry Students, J. Chem. Educ., 60(1), 25–26.
- Arthur P., Ludwig M., Castelli J., Kirkwood P. and Attwood P., (2016), Prepare, Do, Review: a skills-based approach for laboratory practical classes in biochemistry and molecular biology, Biochem. Molec. Biol. Educ., 44(3), 276–287.
- Bowen C. W., (1999), Development and score validation of a Chemistry Laboratory Anxiety Instrument (CLAI) for college chemistry students, Educ. Psychol. Meas., 59(1), 171–185.
- Buntine M. A., Read J. R., Barrie S. C., Bucat R. B., Crisp G. T., George A. V., Jamie I. M. and Kable S. H., (2007), Advancing Chemistry by Enhancing Learning in the Laboratory (ACELL): a model for providing professional and personal development and facilitating improved student laboratory learning outcomes, Chem. Educ. Res. Pract., 8(2), 232–254.
- Chittleborough G. D., Mocerino M. and Treagust D. F., (2007), Achieving greater feedback and flexibility using online pre-laboratory exercises with non-major chemistry students, J. Chem. Educ., 84(5), 884–888.
- Cooper B. and Foy J. M., (1967), Evaluating Effectiveness of Lectures, Univ. Quart., 21(2), 182–185.
- Elliott M. J., Stewart K. K. and Lagowski J. J., (2008), The role of the laboratory in chemistry instruction, J. Chem. Educ., 85(1), 145–149.
- Foertsch J., Moses G., Strikwerda J. and Litzkow M., (2002), Reversing the Lecture/Homework Paradigm Using eTEACH® Web-based Streaming Video Software, J. Eng. Educ., 91(3), 267–274.
- Galloway K. R., Malakpa Z. and Bretz S. L., (2016), Investigating Affective Experiences in the Undergraduate Chemistry Laboratory: Students' Perceptions of Control and Responsibility, J. Chem. Educ., 93(2), 227–238.
- Hofstein A. and Lunetta V. N., (1982), The Role of the Laboratory in Science Teaching – Neglected Aspects of Research, Rev. Educ. Res., 52(2), 201–217.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: foundations for the twenty-first century, Sci. Educ., 88(1), 28–54.
- Jones K. B. and Gellene G. I., (2005), Understanding attrition in an introductory chemistry sequence following successful completion of a remedial course, J. Chem. Educ., 82(8), 1241–1245.
- Kurbanoglu N. I., (2014), Development and Evaluation of an Instrument Measuring Anxiety toward Biology Laboratory Classes among University Students, J. Balt. Sci. Educ., 13(6), 802–808.
- O'Flaherty J. and Phillips C., (2015), The use of flipped classrooms in higher education: a scoping review, Internet High. Educ., 25, 85–95.
- Reid N. and Shah I., (2007), The role of laboratory work in university chemistry, Chem. Educ. Res. Pract., 8(2), 172–185.
- Schmid S., Youl D. J., George A. V. and Read J. R., (2012), Effectiveness of a Short, Intense Bridging Course for Scaffolding Students Commencing University-level Study of Chemistry, Int. J. Sci. Educ., 34(8), 1211–1234.
- Taber K. S., (2009), Learning at the Symbolic Level, in Gilbert J. K. and Treagust D. (ed.) Multiple Representations in Chemical Education, Dordrecht, Netherlands: Springer, pp. 75–105.
- Teo, T. W., K. C. D. Tan, Y. K. Yan, Y. C. Teo and L. W. Yeo (2014), How flip teaching supports undergraduate chemistry laboratory learning, Chem. Educ. Res. Pract., 15(4), 550–567.
| This journal is © The Royal Society of Chemistry 2017 |