Experimental and theoretical study of intramolecular O⋯O interaction in structurally rigid β-keto carboxylic esters†
Chiranjeev Sharmaa,
Ashawani K. Singha,
Jyothish Joyb,
Eluvathingal D. Jemmisc and
Satish K. Awasthi*a
aChemical Biology Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: skawasthi@chemistry.du.ac.in; satishpna@gmail.com; Tel: +91-95812087608
bIndian Institute of Science Education and Research (IISER), Thiruvananthapuram, 695016 Kerala, India
cDepartment of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore-560 012, India
First published on 19th September 2016
Abstract
Here we report the crystal structures of quinolone carboxylate and bisethoxycarbonylvinylaniline derivates containing an O⋯O distance shorter than the sum of their van der Waals radii, which can be ascribed to their steric demand per se, which provide unequivocal evidence of intramolecular 1,5-closed shell type interaction. Theoretical studies including Quantum Theory of Atoms in Molecules (QTAIM) and Natural Bond Orbital (NBO) analysis are employed to characterize the nature of the closed shell O⋯O interaction. We found that the lone pair electrons on the interacting oxygens undergo stabilization due to negative hyperconjugation and maintains the otherwise repulsive O⋯O close contact.
Introduction
Our current understanding and one of the most consistent observation suggests that the non-bonded intramolecular interaction between two oxygen atoms within a molecule exert a destabilizing force1 with mechanistic implications.2 An extensive literature survey reveals that despite a large number of publications on important bioactive structurally rigid molecules (1–4) containing O⋯O distances still shorter than the sum of van der Waals radii, the existence of a non-bonded 1,5-type O⋯O interaction is either neglected or considered unremarkable (Fig. 1).3–5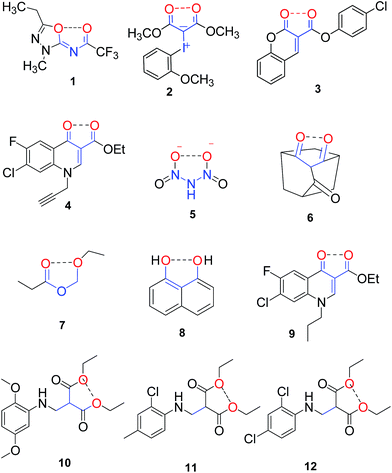 | ||
| Fig. 1 Non bonded O⋯O interaction in geometrically restricted organic molecules (O⋯O distance < 3.1 Å). | ||
Although unusual, O⋯O interaction in the dinitramide anion (5) have been characterized as bonding, closed shell type, using experimental and computational electron density analysis.6 Interestingly, the contribution from non-bonded oxygen–oxygen interactions towards the enthalpy of formation of 6 and 7 was reported to produce stabilization effect.7,8 In addition, atomic calculations by Pakiari and Eskandari in 2007 revealed that O⋯O interaction can impart as much as 11 kcal mol−1 (in B3LYP) and 16 kcal mol−1 (in MP2) of local stabilization to the total energy in 1,8-naphthalenediol (8).9 Later Jablonski et al. disproved these results and concluded that such interactions are repulsive in nature.10,11 Das et al., has recently reported the unexpected O⋯O interactions between the oxygen atoms of the coordinated aldehyde groups in a mononuclear cobalt(III) Schiff base azide complex using several computational tools, including Bader's “atoms-in-molecules” (AIM) and natural bond orbital (NBO) analyses.12 In view of the ambiguity seen in the nature of the O⋯O non-bonded interaction, we present our results on the β-keto carboxylic esters of the quinolone family which gives an insight into the origin of the attractive or repulsive nature of O⋯O interactions.
Results and discussion
While developing fluoroquinolones for antiplasmodial activity as a part of continuing research work on synthesis of new antimalarials13 and characterization of small organic molecules for the evaluation of rare and unusual interactions by X-ray crystal studies,14 we recognized the non-bonded O⋯O distances between the oxygen atoms of the keto group and the carboxylic ethyl ester group at C4 and C3 positions in quinolone derivative (9) significantly shorter than the sum of the corresponding van der Waals radii. It is well established that carbonyl at C4 is essential and limited substitution is permitted at positions 2 and 3 for significant biological activity in quinolones.15 In addition to O1⋯O2 close contact in quinolones, the carboxylic/ester group show coplanarity with the adjacent ring due to conjugation which is shown by their torsion angles.16 This enables metal ions to coordinate with quinolone and render biological activity. Further, aromatic chelators like 8-hydroxyquinoline and 2-mercaptopyridine-N-oxide capable of forming non-ionized complex with divalent metallic ions are also reported to possess antimalarial activity due to chelation.17 Hence, developing chemotherapeutic approaches with attention to such structural rationalization may prove fruitful in combating the world's most severe health problem i.e., malaria.18 Despite the wealth of experimental work on quinolones19 and availability of literature on chalcogen bonds,20,21 the intramolecular O⋯O interactions in quinolones has not been understood well.Based on the forgoing observation, we reasoned that the crystal structure of geometrically restricted organic molecules possessing β-keto/carboxylic ester functionality should also exhibit similar interactions. Compounds 10–12 were then synthesized, characterized and crystallized. We chose hexane as a solvent for crystallization because the use of the polar solvent might inhibit the intramolecular interaction by solvation. Compounds 9–12 crystallized in P21/n, P21/n, P212121 and P21/n space groups respectively with four molecules each per unit cell. The crystal data of all four molecules are solved as error free non-disordered structures (Fig. 2).
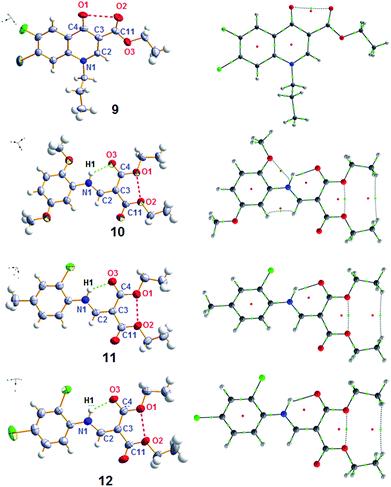 | ||
| Fig. 2 Crystal structures and molecular graphs of 9–12. Green and red dots in the molecular graph indicate BCP and RCP respectively. | ||
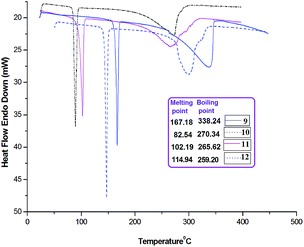
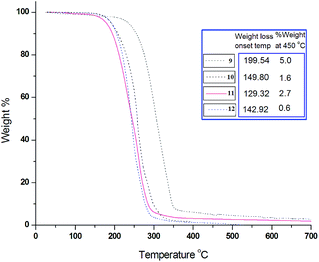
Further, there is no solvent inclusion in the crystal lattice, thermal studies (Fig. 3 and 4) also corroborates to it. Sharp endothermic peaks in DSC thermogram corresponding to the melting points of compounds 9–12 infer the purity of the sample and good degree of crystallinity. TGA curve exhibits single step weight loss for all compounds suggesting no solvent inclusion. Further, complete loss of weight in TGA occurs (only >5% residual compound remaining) accompanied by the endothermic transition in the DSC. It could be ascribed to the evaporation, i.e., the boiling point of these compounds.
The crystal structures of 9–12 were found to be stabilized by intermolecular C–H⋯π, C–Cl⋯π and hydrogen bonding interactions in the crystal lattice (Fig. S1–S4†). Interestingly, in bisethoxycarbonyl-vinylanilinederivates 10–12, intramolecular hydrogen bonding also stabilizes, strengthens and locks one carboxylic handle to facilitate the intramolecular O⋯O interactions. The compounds also possess a planar conformation and low torsion angles along O1–C4–C11–O2. The highly unfavorable O⋯O close contact with small torsional angle between the interacting carbonyl groups (O1–C4–C11–O2) is surprising. These interactions could emerge as a result of the overall packing of the molecules in the crystal lattice.22 To verify this, geometry optimization of the free standing gas phase molecules 9–12 were carried out (see ESI† for more details). The general notion that the close packing is the main reason for O⋯O close contact was found to be true for compounds 10–12, because the geometry optimization leads to large dihedral angle deviation (≈20°), Table 1. To our surprise, compound 9 retained the crystal geometry with only a slight change in the O1–C4–C11–O2 torsion angle (1.99°). This observation led us to believe the existence of a chain of restoring forces at the O⋯O contact region in 9 that overweighs the lone pair–lone pair repulsion experienced in 10–12. The Cambridge Structural Database (CSD) search also support the fact that most of the crystal structures with short O⋯O contact possess the interaction distance of 2.8 Å with an average dihedral angle more than 10° (Fig. S5†).23
| O1⋯O2 (Å) | O1–C4–C3 (°) | C3–C11–O2 (°) | χa (°) | |
|---|---|---|---|---|
| a χ is the torsion angles between O1–C4–C11–O2. | ||||
| 9 | 2.847 | 126.01 | 125.91 | −4.84 |
| (2.842) | (125.88) | (125.96) | (−6.81) | |
| 10 | 2.580 | 115.68 | 115.81 | 7.26 |
| (2.622) | (115.63) | (114.43) | (18.66) | |
| 11 | 2.516 | 115.83 | 113.71 | −1.71 |
| (2.627) | (115.62) | (114.28) | (−20.07) | |
| 12 | 2.539 | 115.44 | 114.51 | 2.32 |
| (2.629) | (115.59) | (114.25) | (20.193) | |
QTAIM analysis of the compounds 9–12 showed the existence of a bond path (BP) and bond critical point (BCP) connecting the interacting oxygens O1 and O2. Topological parameters are validated by comparing with a well-established compound dinitramide anion-5.6 The density (0.011–0.016) and Laplacian of density (0.046–0.070) values at the O1⋯O2 BCP are consistent with the criteria proposed by Koch and Popelier for weak interactions.24 The positive value of Laplacian of density at the BCP verifies the nature of interaction as closed shell, Table 2.
| Compound | ρBCP | ∇2ρ | λ1 | λ2 | λ3 | ε | GBCP | VBCP | HBCP | δ(O⋯O) |
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | +0.0118 | +0.0463 | −0.0096 | −0.0048 | +0.0607 | +1.0032 | +0.0105 | −0.0094 | +0.0011 | 0.0607 |
| 10 | +0.0157 | +0.0701 | −0.0147 | −0.0131 | +0.0979 | +0.1251 | +0.0153 | −0.0130 | +0.0023 | 0.0724 |
| 11 | +0.0156 | +0.0692 | −0.0145 | −0.0130 | +0.0967 | +0.1188 | +0.0151 | −0.0129 | +0.0022 | 0.0716 |
| 12 | +0.0155 | +0.0687 | −0.0144 | −0.0129 | +0.0961 | +0.1166 | +0.0287 | −0.0263 | +0.0024 | 0.0711 |
| 5 | +0.0201 | +0.0876 | −0.0183 | −0.0159 | +0.1219 | +0.1471 | +0.0192 | −0.0165 | +0.0027 | 0.0853 |
The delocalization index, δ(O⋯O), a measure of the average number of electrons delocalized between the interacting atoms, is in accordance with the values of compound 5. A ring critical point (RCP) was also found in the O1–C4–C3–C11–O2 five-membered pseudo ring of compounds 9–12. The near zero value of λ2 and nearby placement of BCP and RCP in 9 suggests an instability at the O⋯O interaction region compared to that in compounds 10–12. It is directly reflected in the high bond ellipticity value (1.0032) of BCP for compound 9.
QTAIM analysis showed the existence of weak intramolecular interaction between the non-bonded O1 and O2 in compounds 9–12. In addition, the Non-Covalent Interaction (NCI) plots also revealed the nature of interaction at the O⋯O contact region as van der Waals/non-repulsive (Fig. S8†).25 However, one factor still unclear is the geometrical preference (short dihedral angle) of compound 9 in comparison with 10–12. Isodesmic reactions10,26 were employed to measure the interaction energy at the O⋯O contact of compound 9, Scheme 1. Since the left hand side of the reaction involves the breakage of one C–C, one C–H, and the weak O⋯O contact, and the right hand side involves the formation of one C–C and one C–H bonds, the reaction energy should in principle represent the strength of the O⋯O contact. Calculated value of reaction energy (3.17 kcal mol−1) indicates the presence of a destabilizing interaction at the O⋯O region. Hence, we devote our further study for the detailed analysis of the geometrical preference in compound 9 that holds the destabilizing O1⋯O2 contact at short dihedral angles.
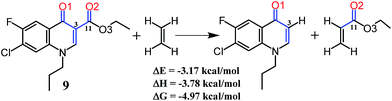 | ||
| Scheme 1 Isodesmic reactions for the evaluation of O⋯O intramolecular interaction energy of compound 9. | ||
The nature of lone pairs–lone pair interaction at the weak O⋯O contact region of compound 9 has further been studied with the help of NBO analysis. Studies were carried out using three different DFT functionals (M06-2X, CAM-B3LYP, ωB97XD) in conjunction with three different basis sets (6-311+g(d,p), Aug-cc-pVTZ, Def2-TZVP). Consistent results are obtained from all levels of theory, Tables S4 and S5.† Here, we use the results from M06-2X/6-311+g(d,p) level of theory for further discussion. Computations revealed the existence of induced negative hyperconjugation27,28 at the interacting oxygens that minimizes the lone pair repulsion. Negative hyperconjugation delocalizes the oxygen lone pair to its neighboring σ*/π* bond-orbital as shown in Fig. 5. Here we use compound 9 and its higher energy isomer 13 (obtained by the rotation of O1–C4–C11–O2 torsion angle of 9 by 66.34°) with no direct O⋯O interaction to substantiate our argument. Table S4† summarizes the most prominent stabilization interactions of all the three oxygen atoms of 9 in comparison with conformer 13. Only two negative hyperconjugative stabilizations showed drastic change in magnitude as illustrated in Fig. 5. The interaction of lone pair on O1 with the neighbouring C–C bonds is least affected by the change in geometry from 9 and 13. However, the influence of lone pair on O1 to the negative hyperconjugation on O2 is substantially large. In compound 9, incoming electron density from O3 to the C11![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2 π* orbital diminishes (6.83 kcal mol−1) due to the electronic repulsion from O1 lone pair. While the donation from O2 to the C11–O3 σ* increases (3.53 kcal mol−1) as compared to 13. Here, O1 enforces an induced negative hyperconjugative donation of electron density from O2 to other parts of the molecule and also discourages the intake of extra electron density towards C11
O2 π* orbital diminishes (6.83 kcal mol−1) due to the electronic repulsion from O1 lone pair. While the donation from O2 to the C11–O3 σ* increases (3.53 kcal mol−1) as compared to 13. Here, O1 enforces an induced negative hyperconjugative donation of electron density from O2 to other parts of the molecule and also discourages the intake of extra electron density towards C11![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2, which results in diminished lone pair–lone pair repulsion at the O1⋯O2 contact region.
O2, which results in diminished lone pair–lone pair repulsion at the O1⋯O2 contact region.
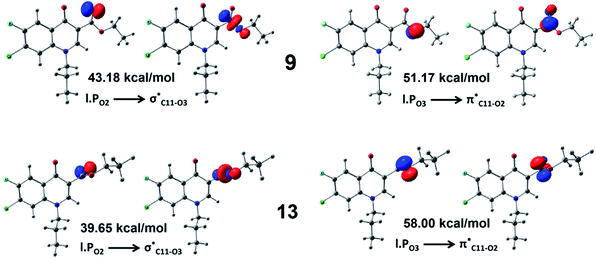 | ||
| Fig. 5 Negative hyperconjuative stabilization of lone pair on O2 and O3 with neighbouring bond orbitals in compounds 9 and 13 obtained from NBO calculations at M06-2X/6-311+g(d,p) level of theory. | ||
The removal of extra electron density at the weak contact region led to short dihedral angle. More electron density can be removed from the O1⋯O2 region by replacing CH2 group in the C2H5OCO arm of 9 with CF2 group, and it lead to very small torsional angle of 2.62° (Fig. S9†). Further proof can be obtained by blocking the internal electron flow in 9 by replacing O3 with CH2 group. It leads to the torsional angle flip of 20.80° at the O1–C4–C11–O2 (Fig. S10†). Hence, any strategy to decrease the lone pair electron density at the interacting oxygens through negative hyperconjugation would be desirable to stabilize these compounds with a shorter torsional angle.
Conclusions
In summary, our study deepens the understanding about the nature of bonding of the less explored intramolecular O⋯O contact regions. The minimal lone pair repulsion at the weak contact region achieved by the induced local negative hyperconjugation along with favorable orbital interaction is the primary reason for the remarkable structural stability of the compound 9. It can be ascertained from the present study that presence of an electron withdrawing group at the α-carbon of the interacting oxygen is necessary for the stabilization of compounds containing O⋯O close contact at short dihedral angles. We propose that the effective delocalization of the interacting lone pairs to other parts of the molecule is a better strategy to bring electronegative atoms closer with diminished torsional strain at the contact region.Acknowledgements
CS, AKS and JJ would like to thank UGC, CSIR and UGC respectively for SRF. EDJ thanks DST for funding through the J. C. Bose Fellowship. The financial assistance from University of Delhi and University Scientific Instrumentation Center (USIC), University of Delhi, Delhi-110007, India for analytical data are also acknowledged. The authors thank IISER-TVM and IISc-Bangalore for computational facilities.References
- M. A. McCarrick, Y. D. Wu and K. N. Houk, J. Am. Chem. Soc., 1992, 114, 1499–1500 CrossRef CAS.
- R. N. Warner, G. M. Elsey, L. Maksimovic and M. R. Johnston, Tetrahedron Lett., 1995, 36, 7753–7756 CrossRef.
- C. Zhu, A. Yoshimura, L. Ji, Y. Wei, V. N. Nemykin and V. V. Zhdankin, Org. Lett., 2012, 14, 3170–3173 CrossRef CAS PubMed.
- X. Q. Guo, J. Yan, Y. Gan, Q. Song and X. J. Gou, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o574 CAS.
- Y. Nagao, T. Hirata, S. Goto, S. Sano, A. Kakehi, K. Iizuka and M. Shiro, J. Am. Chem. Soc., 1998, 120, 3104–3110 CrossRef CAS.
- E. A. Zhurova, V. G. Tsirelson, A. I. Stash and A. A. Pinkerton, J. Am. Chem. Soc., 2002, 124, 4574–4575 CrossRef CAS PubMed.
- M. Masson, Acta Chem. Scand., Ser. B, 1974, 28, 895–899 CrossRef.
- M. Masson, Acta Chem. Scand., Ser. B, 1974, 28, 905–908 CrossRef.
- A. H. Pakiari and K. J. Eskandari, Mol. Struc-Theochem, 2007, 806, 1–7 CrossRef CAS.
- M. Jabłoński, J. Phys. Chem. A, 2012, 116, 3753–3764 CrossRef PubMed.
- M. Jabłoński and M. Palusiak, Chem. Phys., 2013, 415, 207–213 CrossRef.
- M. Das, B. N. Ghosh, A. Bauza, K. Rissanen, A. Frontera and S. Chattopadhyay, RSC Adv., 2015, 5, 73028–73039 RSC.
- (a) S. K. Dixit, N. Mishra, M. Sharma, S. Singh, A. Agarwal, S. K. Awasthi and V. K. Bhasin, Eur. J. Med. Chem., 2012, 51, 52–59 CrossRef CAS PubMed; (b) N. Yadav, C. Sharma and S. K. Awasthi, RSC Adv., 2014, 4, 5469–5498 RSC.
- (a) C. S. Neupane and S. K. Awasthi, Tetrahedron Lett., 2012, 53, 6067–6070 CrossRef CAS; (b) C. Sharma, J. Joy, M. Nethaji, E. D. Jemmis and S. K. Awasthi, Asian J. Org. Chem., 2016 DOI:10.1002/ajoc.201600330.
- A. Wohlkonig, P. F. Chan, A. P. Fosberry, P. Homes, J. Huang, M. Kranz, V. R. Leydon, T. J. Miles, N. D. Pearson, R. L. Perera, A. J. Shillings, M. N. Gwynn and B. D. Bax, Nat. Struct. Mol. Biol., 2010, 17, 1152–1153 CAS.
- M. L. Glowka, D. Martynowski, A. Olczak, J. Bojarska, M. Szczesio and K. Kozlowska, J. Mol. Struct., 2003, 658, 43–50 CrossRef CAS.
- L. W. Scheibel and A. Alder, Mol. Pharmacol., 1980, 18, 320–325 CAS.
- WHO, World Malaria Report 2015, http://www.who.int/malaria/publications/world-malaria-report-2015/en/.
- I. Turel, Coord. Chem. Rev., 2002, 232, 27–47 CrossRef CAS.
- D. B. Werz, R. Gleiter and F. Rominger, J. Am. Chem. Soc., 2002, 124, 10638–10639 CrossRef CAS PubMed.
- C. Bleiholder, D. B. Werz, H. Köppel and R. Gleiter, J. Am. Chem. Soc., 2006, 128, 2666–2674 CrossRef CAS PubMed.
- J. Dunitz, IUCrJ, 2015, 2, 157–158 CrossRef CAS PubMed.
- CSD, version 5.36/Conquest1.17, November 2014 Search PubMed.
- U. Koch and P. L. A. Popelier, J. Phys. Chem., 1995, 99, 9747–9754 CrossRef CAS.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- W. J. Hehre, R. Ditchfield, L. Radom and J. A. Pople, J. Am. Chem. Soc., 1970, 92, 4796–4801 CrossRef CAS.
- I. Fleming, Molecular Orbitals and Organic Chemical Reactions, Wiley, Chichester, West Sussex, U.K., 2009 Search PubMed.
- A. D. McNaught and A. Wilkinson, Compendium of Chemical Terminology: IUPAC Recommendations, Blackwell Science, England, Malden, MA, USA, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 915054, 916682, 920032 and 920439. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra20483j |
| This journal is © The Royal Society of Chemistry 2016 |