Noble gas bound beryllium chromate and beryllium hydrogen phosphate: a comparison with noble gas bound beryllium oxide†
Abstract
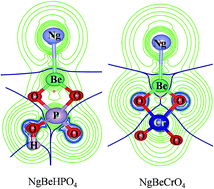
A comparative study is made on the noble gas (Ng) binding ability of beryllium hydrogen phosphate (BeHPO4), beryllium chromate (BeCrO4), and beryllium oxide (BeO) via density functional theory and ab initio calculations. BeO serves as a prototype example of a Be based Lewis acid with remarkable Ng binding capability. Although NgBeHPO4 and NgBeCrO4 have lower Ng–Be bond dissociation energy by 1.4–4.6 and 2.4–6.3 kcal mol−1, respectively, than NgBeO, the corresponding free energy changes at the standard state show that Ar–Rn analogues may be viable even at an ambient condition. The nature of bonding in all these Ng bound complexes is exactly the same, being exclusively a donor–acceptor type of interaction as indicated by the natural bond orbital, electron density and energy decomposition analyses (EDA) in conjunction with natural orbitals for chemical valence calculations. The negative local energy density values at the bond critical points of Ng–Be bonds involving Kr–Rn imply the covalent nature of the bonding which is further supported by the dominant orbital contribution (80–88%) towards the total stabilization as obtained from the EDA. In fact, the variation in the orbital term is responsible for the observed trend of their Ng binding ability in changing either the Ng atoms or the Be system. Further, Ng → BeY (Y = HPO4, CrO4, O) σ-donation is the key contributor (70–82%) of the orbital term, whereas Ng ← BeY π-back donation is responsible only for 15–21% of the total orbital interaction.

 Please wait while we load your content...
Please wait while we load your content...