Mechanical oscillation of dynamic microtubule rings†
Abstract
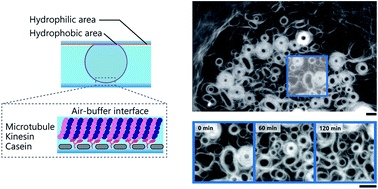
Mechanical oscillation is a ubiquitous phenomenon observed in living systems, which emerges from a wide range of well-organized self-assembled structures, and plays important roles in many biological processes. Although considerable efforts have been devoted to demonstrate the mechanical oscillation of organized structures produced through self-assembly in vitro, it has rarely been documented. Here we report the mechanical oscillation of ring-shaped structures, composed of multiple microtubule (MT) filaments, obtained through energy dissipative self-assembly of MT filaments at an air-buffer interface. The MT rings exhibit autonomous oscillation manifested through periodic changes in the size and shape. We propose the oscillation of the MT rings is attributed to mechanical feedback arising from accumulated stress induced by the driving force of the motor protein system. This work might offer new insights to our current understanding on the mechanical feedback driven oscillation of organized structures and its effect on dynamic processes in living systems.

 Please wait while we load your content...
Please wait while we load your content...