Performance of a nickel–alumina catalytic layer for simultaneous production and purification of hydrogen in a tubular membrane reactor
Abstract
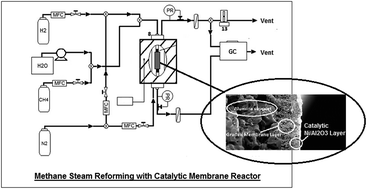
A catalytic membrane reactor was synthesized by coating a 4–5 micron thick Ni/γ-Al2O3 layer on top of a hydrogen selective SiO2/Al2O3 composite membrane using a sol–gel method. Mercury intrusion and BET analysis indicated a uniform size distribution with an average pore size of 285 nm and average surface area of 279 m2 g−1. Single-component permeation tests were carried out for H2, CH4 and CO2 in the temperature range of 650–800 °C and the results showed the same permeance and selectivity values for hydrogen as the composite membrane without a catalytic layer. Performance of the catalytic membrane was evaluated by using as a membrane reactor for the methane steam reforming reaction with a C : H molar ratio of 1 : 3 at a gas hourly space velocity (GHSV) of 100 000 h−1 and 3 bar. CH4 conversion increased from 52% to 91% with increasing reaction temperature from 600 °C to 750 °C, which is well above the equilibrium curve at the reaction conditions, but slightly lower than the membrane reactor with a packed nickel catalytic bed because of its higher surface area compared to the catalytic layer. Hydrothermal stability of the catalytic membrane reactor was evaluated in a reforming reaction at 650 °C. Hydrogen permeance dropped by only 45% from 5.0 × 10−7 mol m−2 s−1 Pa−1 to 2.7 × 10−7 mol m−2 s−1 Pa−1 after exposure to a humid atmosphere for 48 h, which means no major morphological changes in the catalytic membrane's structure.

 Please wait while we load your content...
Please wait while we load your content...