Inoculation of bacteria for the bioremediation of heavy metals contaminated soil by Agrocybe aegerita†
Xue Lia,
Shunwen Dongb,
Yuan Yaoa,
Wenjin Shia,
Minghui Wua and
Heng Xu*a
aKey Laboratory of Bio-resources and Eco-environment (Ministry of Education), College of Life Sciences, Sichuan University, Chengdu, Sichuan 610064, China. E-mail: xuheng64@sina.com; Fax: +86 28 85418262; Tel: +86 28 85414644
bIndustrial Crop Research Institute of Sichuan Academy of Agricultural Sciences, Chengdu, Sichuan 610300, China
First published on 1st July 2016
Abstract
The combination of mushrooms and bacteria was used as a novel technique to remediate soils polluted by heavy metals. Pot experiments were conducted to investigate combined effects of Agrocybe aegerita and Serratia spp. on Ni and Cd polluted soils. The impacts of single inoculation and co-inoculation of Serratia spp. on the growth of A. aegerita, bacterial counts, accumulation of Ni and Cd by A. aegerita, heavy metal speciation and enzymatic activities in soil, along with bacterial community structure were studied. The results indicated that mushroom biomass was promoted by both solitary inoculation and co-inoculation. The increase of bacterial numbers and soil enzymatic activities, and the greater amount of heavy metals in A. aegerita also showed that these combinations could alleviate the heavy metals stress more energetically. Simultaneously, proportions of HOAc extractable and residual metals in soil with A. aegerita and bacteria were respectively higher and lower than those in other treatments, supporting the idea that combinations could activate heavy metals better. Furthermore, the high throughput Illumina pyrosequencing analysis revealed the impacts of different treatments on the bacterial community structure and composition, reinforcing that the integration of bacteria and mushroom was an effective method for bioremediation of soil with heavy metals.
1 Introduction
With the discharge of the industrial wastes and sewage irrigation, extensive use of pesticides, herbicides, and fertilizers, the safety of terrestrial and aquatic ecosystems is seriously affected.1,2 Meanwhile, heavy metals are apt to accumulate through food chain, and consequently, inducing potential human health problems.3 Given that soil plays an important role in sustaining plant and animal productivity, the protection of soil quality is critically important. Much research has attached great importance to this problem. The effect of single heavy metals on the soil was studied extensively.4–6 Yet compound heavy metal pollution is becoming more serious with each passing day. Cd and Ni are heavy metals of great concern in agricultural ecosystems because they have been reported to be toxic to soil microorganisms, crops and human beings.7 Since heavy metals cannot be biodegraded, various physicochemical and biological methods have been developed to remove heavy metals from soils.Phytoremediation is recognized as a simple, cost-effective and self-sustainable technology for the detoxification of heavy metal contaminated sites.8 In recent years, the use of metal resistant plant growth-promoting bacteria (PGPB) or plant growth-promoting rhizobacteria (PGPR) has attracted much attention as they can affect heavy metal mobility and improve plant growth.5,6,9 Meanwhile, research on co-inoculations of bacteria found that co-inoculation led to higher heavy metal accumulation in plants than inoculation with single bacterium.10
Apart from green plants, mushrooms are also well documented to bio-accumulate heavy metal ions.4 Remediation by mushrooms may become a promising bioremediation technology due to their ease of cultivation in fields, fast growth and large amount of biomass11 and the remediation of contaminated soil with heavy metals by mushrooms and bacteria is a novel technique.12 It is reported that the addition of microbes could enhance the mushrooms growth and metal accumulation.12,13 However, most of the investigations to date focused on mushroom growth14 or mycelia growth15 or just the heavy metal mobilizing features of PGPB,13,15 rather than the development of the contaminated soil and soil bacterial community structure. The high sensitivity of soil microorganisms to environmental changes16 and microbial community responses to environmental stresses are critical for microbial growth, survival and adaptation.17 Consequently, these parameters are of great importance to assess whether a bio-remediation technology is successful.16
Agrocybe aegerita is an edible mushroom with high contents of proteins and investigators found that it could grow very well in lead polluted soil.18 The lack of information on remediation on compound heavy metal contaminated soils by A. aegerita has increased the need for the improvement of bacteria for the remediation of the same.
The current study aims to investigate: (1) bioremediation of A. aegerita for Cd & Ni compound contaminated soil and the influence of PGPB on growth, heavy metals accumulation of A. aegerita; (2) solitary inoculation and co-inoculation of bacteria on the improvement of soil enzymatic activities and soil bacterial community structure; (3) potential of A. aegerita with bacteria to remediate compound heavy metals contaminated soils.
2 Material and methods
2.1 Characterization of metal mobilizing strain
2.2 Experiment design
Since these strains showed different resistance to Ni and Cd, the aim of present study was to examine the impacts of co-inoculation of FFC5 and CG8 as well as solitary inoculation of H3 on A. aegerita along with their effects on the soil. The experimental designs are shown in Table 1. All these groups were tested with three replications.| T1 | T2 | T3 | T4 | T5 | CK | |
|---|---|---|---|---|---|---|
| a +: the ingredient(s) was/were contained. −: the ingredient(s) was/were not contained. | ||||||
| A. aegerita | + | + | − | − | + | − |
| FFC5 + CG8 | + | − | + | − | − | − |
| H3 | − | + | − | + | − | − |
2.3 Soil preparation and pot experiment
Soils collected from the campus area of Sichuan University, Chengdu, China were dried and passed through a 2 mm sieve, the main characteristics of the soil were as follows: pH 6.55 ± 0.09, water holding capacity 13.60 ± 0.29%, organic content 17.98 ± 0.28 g kg−1, CEC 10.67 ± 0.44 cmol kg−1, total Cd 0.18 ± 0.02 g kg−1 and total Ni 8.58 ± 0.72 g kg−1. Then, the soil was amended with aqueous solution of NiCl2·6H2O and CdCl2·2H2O to achieve the final concentrations of 100 mg L−1 Ni and 20 mg L−1 Cd. The same amount of deionized water was used in all treatments. Afterward, the spiked soil samples were left in a greenhouse for 2 months prior to the experiment (for metal stabilization). Each pot contained 1.0 kg of the contaminated soil and 0.2 kg of the mycelia bag of A. aegeril; mycelia bags were bought from Shuangliu, Chengdu, China. Moreover, every pot was sprayed with 15 mL bacterial suspensions of selected strains (108 CFU g−1), while 15 mL of sterilized deionized water was used as a control. All these pots received unified water management. About three weeks later, the mature fruiting bodies of A. aegeril were harvested in succession, fresh weights were recorded, then they were washed with deionized water, dried at 60 °C for 2 days in oven and dry weights were also measured.2.4 Heavy metals analysis
The accumulation of Ni and Cd in the mushrooms was determined by flame atomic absorption spectrometry (FAAS) according to Liu et al.19 The sequential extraction of heavy metals in soil was performed using the modified BCR procedure20 with four chemical forms: HOAc extractable, reducible, oxidizable and residual fractions. The brief procedure was that, 1.0 g of soil was shaken at 25 °C, 250 rpm for 16 h with 40 mL of 0.11 M HOAc, then centrifuged for 5 min at 8000 rpm and the supernatant was collected for assay HOAc extractable fraction. The above mentioned residues were shaken at 25 °C, 250 rpm for 16 h with a 40 mL mixture of 0.5 M NH2OH·HCl and 0.05 M HNO3, then centrifuged for 5 min at 8000 rpm and the supernatant was collected for assay in the combination with oxidation fraction. For oxidizable fraction, the above mentioned residues were added to 10 mL 30% H2O2 (pH = 2.5), placed in a water bath at 85 °C for about 1 h until the volume of liquid was <3 mL, then the residue was extracted with 10 mL 30% H2O2 and 50 mL 1.0 M HOAc (pH = 2) was finally added and centrifuged for assay when the volume of liquid was <1 mL. Finally, the above mentioned residual soil was digested with a mixture of 6 mL HNO3, 5 mL HClO4, and 4 mL HF using a microwave digestion method to extract the residual fraction. The different states of heavy metals in the samples were tested by FAAS.2.5 Soil enzyme activity and bacterial counts
Soil samples were collected from each pot on days 0, 7, 14, 21, 28, 45 and 75 to assay soil enzyme activities and bacteria counts. The measurement of dehydrogenase was through spectrophotometry as described by Zhou et al.21 with minor modifications by prolonging the reaction time to 72 h. Activities of fluorescein diacetate (FDA) hydrolysis, catalase, urease, acid phosphatase and invertase were assayed by the methods of Adam and Duncan,22 Johnson and Temple,23 Guan,24 Tabatabai and Bremner,25 and Guan,24 respectively. Dehydrogenase activity was determined spectrophotometrically at 492 nm and expressed as μg triphenylformazan (TPF) per g soil per hour. The activity of FDA was spectrophotometrically determined at 490 nm and presented as the content of fluorescein in dry soil (μg g−1). Catalase activity was determined using a potassium permanganate volumetric method and expressed as potassium permanganate consumption per g soil per hour. Urease activity was spectrophotometrically determined at 578 nm and presented as mg NH4+ per g soil per 24 h. Activities of acid phosphatase and invertase were determined spectrophotometrically at 410 nm and 508 nm, respectively, and individually presented as the production of p-nitrophenol (pNP) in per g soil and mg glucose per g soil per 24 h. Bacterial counts in soil were counted on LB agar medium through the spread-plate method as described by Liu et al.192.6 Bacterial community analysis
The soil bacterial DNA of 5 g soil sample was isolated using a Soil DNA Kit (Omega Bio-tek Inc., Norcross, GA, USA) according to the manufacturer’s instructions. 16S rRNA gene of the extracted DNA was amplified using a pair of primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The bacterial community was investigated on Illumina MiSeq platform, which was conducted by Majorbio Bio-Pharm Technology Co., Ltd (Shanghai, China). Sequences were clustered into operational taxonomic units (OTUs) and the biological information analysis was conducted at 97% similarity by using the Usearch program (version 7.1). Sequences were then phylogenetically assigned to taxonomic classifications using ribosomal database project (RDP) classifier26 and were allocated to genus levels. Hierarchical clustering analysis based on beta diversity distance matrix27 was conducted to describe and compare the similarity and difference between samples.2.7 Statistical analyses and data analyses
Bio-concentration factor (BCF) values of metals from soils to mushrooms were calculated according to the following formula:19The mean and standard deviation values of three replicates were calculated. Statistical significance was evaluated using SPSS 21.0 with ANOVA, and means were compared using least significant differences (LSD) calculated at a significance level of P < 0.05. All figures were performed using the GraphPad Prism 6.0 (USA).
3 Results and discussion
3.1 Mushroom fruiting growth
Stresses of Cd & Ni on mushroom fruiting growth were relieved by co-inoculation of FFC5 and CG8 most (Fig. 1). Comparing with the treatment of non-inoculation, the inoculation of H3 didn’t facilitate the biomass of A. aegerita. However, pot experiments of individual heavy metal pollution (Ni and Cd were 100 and 20 mg L−1, respectively) (ESI Fig. 1†) showed neither single inoculation nor co-inoculation made any differences to the fresh or dry weight of the mushroom when under single Cd stress, and the values were even lower than those of the non-inoculation treatment. It is reported that Cd stress influences auxin homeostasis in plants,28 which may give a reason for above results. In the case of Ni, the fresh weight of A. aegerita increased in different degrees with solitary inoculation or co-inoculation (1.16-folds and 1.48-folds of the control, respectively), and similar outcomes in dry weight of mushroom were observed.The present study indicated that co-inoculation could be more beneficial than solitary inoculation to the growth of A. aegerita under co-contamination of Ni and Cd or single heavy metal nickel stress. The reason may be a considerable release of indole-3-acetic acid (IAA) by co-inoculation, since the production of IAA by PGPB was often directly associated with their potential to stimulate plant growth29 and co-inoculation tended to produce more IAA than individual isolates,30 moreover, bacteria could also act as 1-aminocyclopropane-1-carboxylate (ACC) deaminase producers and phosphate solubilizers which might also enhance the growth of accumulators. In addition, Ni is considered as an essential element primarily because of its function as an irreplaceable component of urease, we speculate that, at this concentration, Ni had limited toxic effects on the plant growth promotion efficiency of microorganisms. With respect to Cd, results were less than satisfactory, which was probably related to the strong physiological toxicity of Cd.
3.2 Microbial numbers and soil enzymes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.60 CFU g−1 soil and log
6.60 CFU g−1 soil and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.11 CFU g−1 soil, respectively). The presence of A. aegerita and co-inoculation was found to stimulate the growth of microorganisms most with an increase in the rate of 16.81%.
6.11 CFU g−1 soil, respectively). The presence of A. aegerita and co-inoculation was found to stimulate the growth of microorganisms most with an increase in the rate of 16.81%.
 | ||
| Fig. 2 Bacterial counts in Ni and Cd co-contaminated soil with different treatments. Error bars represent the standard deviation of three sampled pots. | ||
The results lent some support to previous findings that the growth of microorganisms was stimulated by the presence of mushrooms with or without inoculation of bacteria.19 So also were the results of single heavy metal stress experiment (ESI Fig. 2†). However, T2 performed better in the single heavy metal contaminated soils, yet under combined Cd and Ni stress, the T1 treatment showed excellent improvement.
Soil microbial biomass is closely correlated with the soil ecosystem. It is of great importance to the decomposition of soil organic matter, the fixation and release of nutrients,31 and soil microbes also play an important role in the treatment of organic pollution and heavy metal pollution.32 Various carbohydrates, organic acids, as well as other compounds released by mushrooms, provide a source of nutrients for microbes.33 Lower bacterial counts in unplanted or non-inoculation treatments may be due to the limited nutrients availability19 and lack of symbiotic relationship between the mushrooms and inoculated bacteria. The above results indicated that the combination of microbes and A. aegerita had the ability to alleviate the negative impacts of heavy metals in soil.
A study of the activities of soil enzymes under single heavy metal stress was also conducted to understand the influences of each treatment. ESI Fig. 3 and 4† showed the activities of the six soil enzymes for the period after treatments in single Ni or Cd stress. They shared lots in common with enzymatic activities under Cd–Ni stress, but were much different in some ways. T1 treatment enabled a majority of the soil enzymatic activities most under single Cd stress (ESI Fig. 2†). Otherwise, T2 and T5 treatments also excelled in increasing the activities of FDA, acid phosphatase, dehydrogenase and invertase. Meanwhile, the results were quite different under single Ni stress (ESI Fig. 3†), T2 treatment showed the most positive promotion effects on the soil enzymatic activities. It is noteworthy that some enzymes activities in partial treatments was equivalent to or even lower than that in the control, for instance, urease activity in T1, T3, T4, T5 was similar to that in the control sample. The same situation occurred in the FDA activity of T1, T5; acid phosphatase activity of T4 and T5; dehydrogenase activity of T3, T4 and T5; invertase activity of T3, T4 and T5; catalase activity of T3 and T4.
In the present study, it was clear that violent fluctuation of soil enzymes activities occurred during the days of 0–28 whether it was under compound heavy metal or single heavy metal stress. This is likely due to the increase of the soil microorganism quantities in the beginning, then insufficient rhizospheres led to the death of some microorganisms.9 The treatments inspired the growth of indigenous microorganisms, thus causing serial changes in activities of soil enzymes. During the mid-term and later period of the experiment, soil enzymatic activities in some treatments were activated once again, the possible reasons might be the amendments-induced the growth of resistant microorganisms and changes in the content of available heavy metals in the soil.
| Characters | T1 | T2 | T3 | T4 | T5 | CK |
|---|---|---|---|---|---|---|
| a Correlation is significant at the 0.05 level.b Correlation is significant at the 0.01 level. | ||||||
| Acid phosphatase | 0.577b | 0.516a | 0.39 | 0.704b | 0.711b | 0.489a |
| FDA | 0.582b | 0.565b | 0.594b | 0.291 | 0.343 | 0.676b |
| Dehydrogenase | 0.531a | 0.386 | 0.354 | 0.197 | 0.587b | −0.176 |
| Catalase | 0.495a | 0.818b | 0.630b | 0.759b | 0.848b | 0.517a |
| Invertase | 0.568b | 0.490a | 0.488a | 0.4 | 0.385 | −0.531a |
| Urease | 0.633b | 0.212 | 0.737b | 0.363 | 0.208 | 0.589b |
As one of key enzymes in the soil, dehydrogenase is a good indicator of soil microbial activity.36 However, in present study, the significant correlation coefficient between microbial counts and dehydrogenase was only found in T1 and T5 Treatments. The close relationships of urease, invertase and phosphorus with bacterial biomass in the present study showed their important roles in soil carbon, nitrogen and phosphorus cycling for the growth and multiplication of soil microorganisms.37–39 Catalase activity was related to the bacterial biomass in most treatments since they can prevent cells from damage by reactive oxygen species.40 Affinitive relationships between bacterial biomass and the activities of soil enzymes in the T1 treatment indicated that bacterial activity had a significant effect on the soil enzymes. According to Fig. 2, it was absolutely clear that, at day 75, the microbial biomass in the T1 treatment was significant higher than any other treatments, hence it could be inferred that, the activities of soil enzymes in this treatment must be activated.
However, this phenomenon did not always happen under single heavy metal stress. Under single Cd stress, various enzymes were unrelated to bacterial numbers, and a few were in negative correlations with bacterial numbers (ESI Table 1†). For single Ni stress, soil enzymes activities were only related to bacterial numbers in a few treatments (ESI Table 2†). Inconsistencies in correlations have appeared in previous research.39,41 Many reasons can be used to account for the inconsistencies. Apart from the concentration of the heavy metal in this experiment and stability of enzymes, the reactivity of the heavy metals with specific organic ligands42 is another important item that we have to consider. According to the hard–soft acid base theory, soft acids (such as Cd2+) tend to associate more tightly with soft bases, such as sulphhydryl (R–SH) in proteins, than borderline acids (such as Ni2+).42 And the antibacterial toxicity of these metals is approximately in proportion to their affinity for S.43–45 Besides, in the present study we reconfirmed that soil enzymes can be tracked as indicators of soil quality following the addition of soil amendments. And the combination of A. aegeril and bacteria can actually alleviate heavy metals damage to the soil ecosystem.
3.3 Metal uptake in mushrooms and metal speciation in soil
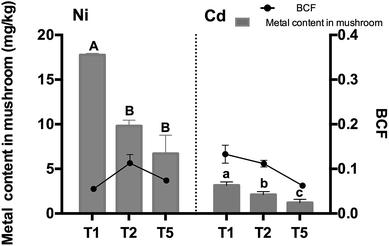
Metal accumulation in mushrooms and bioconcentration factors (BCF) are shown in Fig. 4. The highest Cd and Ni contents were determined as 17.76 ± 0.14 mg kg−1, 3.16 ± 0.29 mg kg−1, respectively. Metal uptake in mushrooms was influenced by the addition of bacteria significantly. Additionally, according to Fig. 4 and ESI Fig. 5,† no matter whether under individual or compound heavy metals stress, Cd accumulation in mushroom remained unchanged in each treatment, yet the content of A. aegerita markedly reduced in co-contamination treatments, with a decreasing rate of 65.74%, 55.68% and 5.47% in T1, T2 and T5, respectively. This phenomenon showed that co-contamination had a notable effect on the addition of microorganisms. The lowest BCF of Cd and Ni by A. aegerita appeared in T5, which were individually 6.03% and 6.7%. Inoculation of PGPB had become a very real possibility, since metal accumulation in mushrooms is related to their chemical forms,19 in addition, microorganisms can change the speciation of metal contaminants.46Fig. 5 shows the metal speciation in the soil. The percentage of HOAc-extractable, reducible, oxidizable and residual Cd were 11.54–14.11%, 64.04–69.79%, 16.55–21.32% and 0.26–2.11%, respectively. T1 and T2 Treatments increased HOAc-extractable and reducible Cd content most remarkably across all the treatments. In the analysis of residual Cd, compared with the control, all the treatments declined notably. In case of Ni, planting with co-inoculation/single-inoculation increased HOAc-extractable Ni by 2.58% and 4.32%, and reduced residual Ni by 7.05% and 6.96% relative to the control, respectively. Heavy metal speciation analysis showed that the difference between the result of single heavy metal stress and that of compound heavy metal stress was tiny (Fig. 5 and ESI Fig. 6†). The increase of HOAc-extractable Ni was conducive to metal translocation from the soil to the mushrooms, however, there was a little difference from previous studies. According to Liu et al.,19 planting mushrooms increased residual Cd, indicating mushroom planting might reduce the damage of Cd to soil microorganisms. The residual fraction of Cd and Ni declined in present study, leading us to speculate that planting with or without inoculation could activate Cd and Ni thus making it more easily absorbed by the mushroom. Moreover, the active state of Cd (99.03%, 99.74% and 97.89% in soil of T1, T2 and T5, respectively) was much higher than that of Ni (66.48%, 66.39% and 59.43% in soil of T1, T2, and T5, respectively), illustrating Cd had more deleterious effects to microbes in soil than Ni, which coincided with the result of the bacterial counts.
Comparing to the percentage of HOAc-extractable, reducible, oxidizable and residual heavy metals in co-contaminated soil, the changes of percentages of each portion in single Cd or Ni contaminated soil shared lots in common. The combination of mushrooms and bacteria increased the HOAc-extractable heavy metal significantly no matter whether under individual or compound heavy metals stress. However, in single heavy metal contaminated soil, the percentage of HOAc-extractable heavy metal was much higher than that in Cd–Ni stress. And this was similar to the reports of Liu et al.19 and Bin Wu et al.,47 since compound heavy metal had a worse impact on the growth of microbes in soil, as well as the mushroom growth. And oxidizable Cd only took up a very small proportion (less than 2.86%) of heavy metal forms in single Cd-contaminated soil, yet oxidizable Cd occupied a larger part (more than 13.00%) of heavy metal forms in co-contaminated soil. But this situation did not happened in the fractions of Ni. The most likely reasons are (1) the differences in metal chemical properties between Cd and Ni, (2) the interactions between heavy metals. In general, the oxidization state of Ni2+ was a common level, while Cd2+ had a high oxidization state.42 This might explain the higher amounts in single Cd stress samples. But in compound heavy metal stress, the appearance of Ni seemed to have a negative effect on the oxidizable Cd.
Heavy metal uptake by mushrooms was mainly related to the metal availability, as well as their ability to transport metals to the aerial part. It is known that polysaccharic components in mushrooms have the property of fixing metallic elements on functional groups. These elements are quickly transported to the cell interior and then circulate throughout the entire mycelium.48 Additionally, mushrooms can secrete various kinds of low-molecular weight organic acids (LMWOAs), which have been suggested to enhance the mobility of heavy metals in soils due to their strong complex affinity with the heavy metals.49,50 Study on physiological mechanisms of heavy metal uptake by hyperaccumulating plants showed that there was a positive correlation between the ability of long distance translocation and enrichment degree of the heavy metal by hyperaccumulators.51 Furthermore, research demonstrated that mushrooms had an excellent performance in this respect.52
With the exception of the innate character of mushrooms, enrichment of heavy metals by mushrooms also occurs in connection with microorganisms in soil, since microbes can not only stimulate accumulators growth,5,6,53 but also affect heavy metal mobility and uptake by accumulators through synthesizing metabolites including siderophores, bio-surfactants, organic acids etc. Besides, some microbes upregulate the expression of extracellular polymers in response to metal stress and these molecules contain functional groups capable of coordinating metal ions.45 These beneficial microorganisms are of interest for phytoremediation, even though they fail to colonize when applied in the field, they can affect soil micro-ecology significantly.54 Our research showed that the presence of bacteria promoted the growth of A. aegerita and heavy metal uptake by mushrooms, accordingly, enhancing bioremediation of heavy metal contaminated soil by mushrooms.
3.4 Bacterial community structure
Four samples were sequenced and compared through the MiSeq sequencing analysis in present study. Sequences with low abundance (less than 1%) were assigned as others. Bacteria from the four samples demonstrated similar diversities but different abundances. 236 different genera out of the total 319 genera types were common to the four libraries. Relative bacterial community abundance on the genus level (Fig. 6) showed that Bacillus and Anaerolineaceae_uncultured were the most different group, and the former comprised approximately 15% (3813) of the OTUs in the CK, which was nearly triple that of T1 (5.59%, 1437 OTUs), T2 (4.66%, 1202 OTUs) and T5 (5.28%, 1357 OTUs) treatments. Previous reports showed that the production of biosurfactants by Bacillus spp. can be a promising application for enhancing bioremediation of sites contaminated with heavy metals.55 Treatments could relieve the abiotic stress to soil microorganisms, which would explain why the abundance of Bacillus was less with treatments. Members of Anaerolineae are known as semi-syntrophic and fatty acids-oxidizing bacteria,56 Anaerolineaceae_uncultured was the most abundant group in the T5 treatment, consisting up to 11.15% (2861) of the OTUs, and was double that of T1 and T2 treatments, yet members of Anaerolineaceae_uncultured in the control (1.36%, 349 OTUs) were found much less than in the treatments. Concurrently, treatments promoted the Flexibacter genus, which was consistent with previous studies.57 In addition, members of Acidimicrobiales_norank, Pseudomonas, as well as Lactococcus were found to be greatly reduced in soil of all treatments, and these bacteria exhibit excellent heavy metal resistance in previous studies,58–60 showing that bioremediation of soils in all treatments was efficient. It is noteworthy that Serratia was only found in the T2 treatment, and it was assigned to Serratia_unclassified on the species level. Though it was a much smaller fraction (0.19%, 49 OTUs), and did not fully demonstrate that Serratia sp. strain H3 sufficiently colonized the soil, it also provided this kind of possibility. The sample dendrogram indicated that T1, T2 and T5 treatments had relatively high impacts on the microbial community structure. And that, compared with T5, both single inoculation and co-inoculation made a difference to the bacterial community structure. | ||
| Fig. 6 Bacterial community structures under different treatments on the genus level. The abundance is presented in terms of a percentage of the total effective bacterial sequences in the sample. | ||
4 Conclusions
In present study, the growth of A. aegerita was enhanced by the presence of Serratia spp. under co-contamination of heavy metals. The combination of A. aegerita and co-inoculation of FFC5 and CG8 significantly increased bacterial counts (16.81%), in accordance with the activities of soil enzymes, including FDA, acid phosphatase, dehydrogenase, catalase, invertase and urease. In addition, both single inoculation and co-inoculation promoted accumulation of Ni and Cd by A. aegerita compared with the control group (40.42% and 165.07% for Ni, respectively; 74.28% and 161.16% for Cd, respectively). Additionally, the combination of A. aegerita and co-inoculation/single inoculation increased HOAc-extractable Ni by 2.58% and 4.32%, and reduced residual Ni by 7.05% and 6.96%, meanwhile, the content of HOAc-extractable Cd increased 2.58% and 2.55%, respectively, and the residual fraction of Cd declined 1.14% and 1.85%, respectively, showing the potential of bacteria in improving the removal of heavy metals from polluted sites. Furthermore, the high throughput Illumina sequencing provided a detailed picture of the bacterial community variations on phylum and genus levels under compound heavy metal pollution. The percentages of bacterial groups in each sample varied at different taxonomic levels, and these variations were to some extent indicative that bioremediation by A. aegerita and bacteria was efficient. The only finding of Serratia_unclassified in the T2 treatment samples also provided a possibility that Serratia sp. strain H3 can colonize heavy metal polluted areas.Acknowledgements
This study was financially supported by the NSFC (No. 41171253, No. J1103518). The authors wish to thank Professor Guanglei Cheng and Dong Yu from Sichuan University for their technical assistance.References
- K. E. Giller, E. Witter and S. P. McGrath, Soil Biol. Biochem., 1998, 30, 1389–1414 CrossRef CAS.
- A. G. Khan, C. Kuek, T. M. Chaudhry, C. S. Khoo and W. J. Hayes, Chemosphere, 2000, 41, 197–207 CrossRef CAS PubMed.
- M. J. McLaughlin, D. R. Parker and J. M. Clarke, Field Crop. Res., 1999, 60, 143–163 CrossRef.
- W. Zhang, Y. Hu, Y. Cao, F. Huang and H. Xu, Chemosphere, 2012, 88, 467–475 CrossRef CAS PubMed.
- Y. Ma, M. Rajkumar, Y. M. Luo and H. Freitas, Chemosphere, 2013, 93, 1386–1392 CrossRef CAS PubMed.
- Y. Ma, M. Rajkumar and H. Freitas, J. Hazard. Mater., 2009, 166, 1154–1161 CrossRef CAS PubMed.
- B. H. Robinson, Sci. Total Environ., 2009, 408, 183–191 CrossRef CAS PubMed.
- I. Raskin, R. D. Smith and D. E. Salt, Curr. Opin. Biotechnol., 1997, 8, 221–226 CrossRef CAS PubMed.
- S. Compant, C. Clement and A. Sessitsch, Soil Biol. Biochem., 2010, 42, 669–678 CrossRef CAS.
- D. F. Rojas-Tapias, R. Bonilla and J. Dussan, Water, Air, Soil Pollut., 2014, 225, 1–13 CrossRef CAS.
- P. G. Miles and S.-t. Chang, Mushroom biology: concise basics and current developments, World Scientific, 1997 Search PubMed.
- J. Jiang, H. Y. Liu, Q. Li, N. Gao, Y. Yao and H. Xu, Ecotoxicol. Environ. Saf., 2015, 120, 386–393 CrossRef CAS PubMed.
- X. B. Jing, N. He, Y. Zhang, Y. R. Cao and H. Xu, Can. J. Microbiol., 2012, 58, 45–53 CrossRef CAS PubMed.
- J. R. Han, Sci. Hortic., 1999, 82, 171–178 CrossRef.
- L. Y. Ji, W. W. Zhang, D. Yu, Y. R. Cao and H. Xu, World J. Microbiol. Biotechnol., 2012, 28, 293–301 CrossRef CAS PubMed.
- D. L. Mummey, P. D. Stahl and J. S. Buyer, Appl. Soil Ecol., 2002, 21, 251–259 CrossRef.
- A. F. Zhou, Z. L. He, Y. J. Qin, Z. M. Lu, Y. Deng, Q. C. Tu, C. L. Hemme, J. D. Van Nostrand, L. Y. Wu, T. C. Hazen, A. P. Arkin and J. Z. Zhou, Environ. Sci. Technol., 2013, 47, 9841–9849 CrossRef CAS PubMed.
- M. Tuomela, K. T. Steffen, E. Kerko, H. Hartikainen, M. Hofrichter and A. Hatakka, FEMS Microbiol. Ecol., 2005, 53, 179–186 CrossRef CAS PubMed.
- H. Y. Liu, S. S. Guo, K. Jiao, J. J. Hou, H. Xie and H. Xu, J. Hazard. Mater., 2015, 294, 121–127 CrossRef CAS PubMed.
- J. H. Li, Y. Lu, H. J. Shim, X. L. Deng, J. Lian, Z. L. Jia and J. H. Li, J. Environ. Monit., 2010, 12, 466–471 RSC.
- Z. R. Zhou, Y. J. Chen, X. Liu, K. Zhang and H. Xu, RSC Adv., 2015, 5, 42768–42776 RSC.
- G. Adam and H. Duncan, Soil Biol. Biochem., 2001, 33, 943–951 CrossRef CAS.
- J. L. Johnson and K. L. Temple, Soil Sci. Soc. Am. J., 1964, 28, 207–209 CrossRef CAS.
- S. Y. Guan, Soil enzyme and its research approaches, China Agriculture Press, Beijing, 1986 Search PubMed.
- M. A. Tabatabai and J. M. Bremner, Soil Biol. Biochem., 1969, 1, 301–307 CrossRef CAS.
- Q. Wang, G. M. Garrity, J. M. Tiedje and J. R. Cole, Appl. Environ. Microbiol., 2007, 73, 5261–5267 CrossRef CAS PubMed.
- X. T. Jiang, X. Peng, G. H. Deng, H. F. Sheng, Y. Wang, H. W. Zhou and N. F. Y. Tam, Microb. Ecol., 2013, 66, 96–104 CrossRef PubMed.
- C. L. Yu, C. D. Sun, C. J. Shen, S. K. Wang, F. Liu, Y. Liu, Y. L. Chen, C. Y. Li, Q. Qian, B. Aryal, M. Geisler, D. A. Jiang and Y. H. Qi, Plant J., 2015, 83, 818–830 CrossRef CAS PubMed.
- O. Stajkovic, D. Delic, D. Josic, D. Kuzmanovic, N. Rasulic and J. Knezevic-Vukcevic, Rom. Biotechnol. Lett., 2011, 16, 5919–5926 Search PubMed.
- S. Chatterjee, G. B. Sau, S. Sinha and S. K. Mukherjee, Arch. Agron. Soil Sci., 2012, 58, 1387–1397 CrossRef CAS.
- S. Roy and J. S. Singh, J. Ecol., 1994, 82, 503–509 CrossRef CAS.
- R. Boopathy, Bioresour. Technol., 2000, 74, 63–67 CrossRef CAS.
- J. I. Rangel-Castro, E. Danell and P. E. Pfeffer, Mycologia, 2002, 94, 190–199 CrossRef CAS PubMed.
- A. V. Ilyina, N. Y. Tatarinova and V. P. Varlamov, Process Biochem., 1999, 34, 875–878 CrossRef CAS.
- F. Cardenas, M. S. de Castro, J. M. Sanchez-Montero, J. V. Sinisterra, M. Valmaseda, S. W. Elson and E. Alvarez, Enzyme Microb. Technol., 2001, 28, 145–154 CrossRef CAS PubMed.
- H. Y. Chu, J. G. Zhu, Z. B. Xie, H. Y. Zhang, Z. H. Cao and Z. G. Li, Aust. J. Soil Res., 2003, 41, 731–739 CrossRef CAS.
- B. H. Byrnes and J. R. Freney, Fert. Res., 1995, 42, 251–259 CrossRef CAS.
- L. Gianfreda, F. Sannino and A. Violante, Soil Biol. Biochem., 1995, 27, 1201–1208 CrossRef CAS.
- E. J. Joner and I. Jakobsen, Soil Biol. Biochem., 1995, 27, 1153–1159 CrossRef CAS.
- J. Katsuwon and A. J. Anderson, Appl. Environ. Microbiol., 1990, 56, 3576–3582 CAS.
- J. C. Tarafdar and H. Marschner, Soil Biol. Biochem., 1994, 26, 387–395 CrossRef CAS.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Nat. Rev. Microbiol., 2013, 11, 371–384 CrossRef CAS PubMed.
- M. L. Workentine, J. J. Harrison, P. U. Stenroos, H. Ceri and R. J. Turner, Environ. Microbiol., 2008, 10, 238–250 CAS.
- D. H. Nies, FEMS Microbiol. Rev., 2003, 27, 313–339 CrossRef CAS PubMed.
- J. J. Harrison, H. Ceri and R. J. Turner, Nat. Rev. Microbiol., 2007, 5, 928–938 CrossRef CAS PubMed.
- E. Baath, Water, Air, Soil Pollut., 1989, 47, 335–379 CrossRef CAS.
- B. Wu, G. Cheng, K. Jiao, W. Shi, C. Wang and H. Xu, Sci. Total Environ., 2016, 562, 732–739 CrossRef CAS PubMed.
- J. A. Campos, N. A. Tejera and C. J. Sanchez, Biometals, 2009, 22, 835–841 CrossRef CAS PubMed.
- D. M. Zhou, C. F. Deng and L. Cang, Chemosphere, 2004, 56, 265–273 CrossRef CAS PubMed.
- E. Gidarakos and A. Giannis, Water, Air, Soil Pollut., 2006, 172, 295–312 CrossRef CAS.
- U. Kramer, J. D. CotterHowells, J. M. Charnock, A. J. M. Baker and J. A. C. Smith, Nature, 1996, 379, 635–638 CrossRef CAS.
- B. Wu, R. Chen, Y. Yao, N. Gao, L. Zuo and H. Xu, RSC Adv., 2015, 5, 67524–67531 RSC.
- B. Lugtenberg and F. Kamilova, Annu. Rev. Microbiol., 2009, 63, 541–556 CrossRef CAS PubMed.
- Y. Xu, G. D. Sun, J. H. Jin, Y. Liu, M. Luo, Z. P. Zhong and Z. P. Liu, J. Hazard. Mater., 2014, 264, 430–438 CrossRef CAS PubMed.
- J. D. Desai and I. M. Banat, Microbiol. Mol. Biol. Rev., 1997, 61, 47–64 CAS.
- T. Narihiro, T. Terada, A. Ohashi, Y. Kamagata, K. Nakamura and Y. Sekiguchi, Water Res., 2012, 46, 2167–2175 CrossRef CAS PubMed.
- S. Llado, A. M. Solanas, J. de Lapuente, M. Borras and M. Vinas, Sci. Total Environ., 2012, 435, 262–269 CrossRef PubMed.
- A. Hassen, Z. Jerboui, M. Cherif, N. Saidi, S. Gharbi and A. Boudabous, Microb. Ecol., 2001, 42, 99–107 CAS.
- L. Li and Y. Ma, J. Dairy Sci., 2014, 97, 5975–5982 CrossRef CAS PubMed.
- S. Mangold, J. Potrykus, E. Bjorn, L. Lovgren and M. Dopson, Extremophiles, 2013, 17, 75–85 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11767h |
| This journal is © The Royal Society of Chemistry 2016 |