Open Access Article
Open Access ArticleTandem Mannich/Diels–Alder reactions for the synthesis of indole compound libraries†
Peng
Wu‡
a,
Michael Åxman
Petersen‡
a,
Rico
Petersen
a,
Thomas
Flagstad
a,
Rachel
Guilleux
b,
Martin
Ohsten
b,
Rémy
Morgentin
b,
Thomas E.
Nielsen
*ac and
Mads H.
Clausen
*ad
aDepartment of Chemistry, Technical University of Denmark, DK-2800, Kgs. Lyngby, Denmark. E-mail: ten@kemi.dtu.dk; mhc@kemi.dtu.dk
bEDELRIS, 115 Avenue Lacassagne, 69003 Lyon, France
cSingapore Centre on Environmental Life Science Engineering, Nanyang Technological University, 637551, Singapore
dCenter for Nanomedicine and Theranostics, Technical University of Denmark, DK-2800, Kgs. Lyngby, Denmark
First published on 5th May 2016
Abstract
A tandem Mannich/Diels–Alder sequence for the synthesis of small-molecule libraries with an indolyl-octahydro-3a,6-epoxy-isoindole core structure is demonstrated in this study. Representative diversification examples based on this scaffold were performed, and a library is being produced within the European Lead Factory (ELF) Consortium.
A large portion of pharmaceutically active compounds and approved drugs,1 including all small-molecule kinase inhibitors approved by the FDA so far,2,3 are structurally dependent on heterocyclic scaffolds.4 The indole core structure is embedded in a plethora of compounds, which exhibit a broad range of biological activities, such as anticancer,5 antibacterial,6 anti-inflammatory,7 and anti-HIV,8 and is clearly one of the most intensively studied heterocyclic scaffolds.9 In fact, indole or fused indole moieties are present in more than 50 FDA-approved small-molecule drugs and countless biologically active compounds currently in clinical or preclinical development.1 Due to the fact that present drug discovery efforts tend to focus on a limited number of scaffolds, there is a growing interest within the chemical and pharmaceutical communities to develop synthetic approaches towards small-molecule libraries that also incorporate new scaffolds.10 Properties relating to lipophilicity, fraction of sp3-hybridised carbon atoms, ratio of chiral/non-chiral centers, and drug-likeness parameters differentiate these newly sought-after scaffolds from most traditional aromatic scaffolds. It is expected that the exploitation of new chemical space represented by these new scaffolds will be associated with novel physico-chemical properties and potentially useful biological effects.11
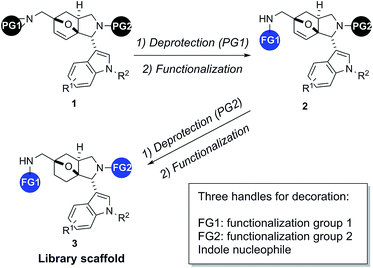
The synthesis of biologically active and structurally diverse small molecule libraries is a current focus of our group.12–14 As a continuation of our efforts to synthesize indole derivatives,15 the amine protected compounds 1 with an indolyl-hexahydroepoxyisoindole core was designed. This scaffold has previously been synthesized employing an intramolecular Diels–Alder reaction16–18 and is virtually unexplored biologically. We envisioned its formation through a convenient tandem Mannich/Diels–Alder reaction sequence. Subsequent cycles of deprotection and functionalization lead to indole compounds 3 with an octahydro-3a,6-epoxyisoindole core and three sites for diversification: one introduced intrinsically by the indole component, in addition to a primary amine and a secondary amine for further decoration (Fig. 1). In the present work, we describe the synthesis of a small-molecule library based on this indolyl-octahydroepoxyisoindole scaffold (3), which combines a bicyclic aromatic indole with a tricyclic aliphatic 3a,6-epoxy-isoindole ring, the latter displaying a high Fsp3 value and several chiral centers.
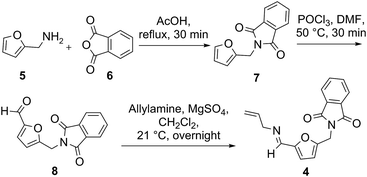
The synthesis of substrate 4 for the Mannich/Diels–Alder reaction was achieved by protection of furfurylamine 5, followed by formylation using phosphoryl chloride in DMF to give aldehyde 8,19 which readily reacted with allylamine to afford the Schiff base 4 in high yield (Fig. 2).
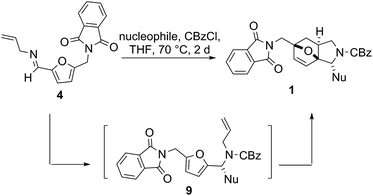
In initial experiments with the one-pot Mannich/Diels–Alder reaction sequence, indole was employed as the C-nucleophile in THF at 70 °C, together with CBzCl, to give the amino-protected compound 1b in 56% yield (entry 1, Table 1). This process is easily applicable to other indole nucleophiles, substituted with either electron-withdrawing or electron-donating groups, affording the corresponding tricyclic 3a,6-epoxyisoindole derivatives 1a and 1c–e in good yields ranging from 59 to 72% (Table 1, entries 1 and 3–6). Attempts to use other C-nucleophilic aromatic systems, such as 1,3-dimethoxybenzene, 1H-pyrrolo[2,3-b]pyridine, 1H-indazole, and thiophene, in this one-pot process led to intractable mixtures with little or no trace of Diels–Alder product (Table 1, entries 6–9). The reaction was monitored by LC-MS, where Mannich intermediates 9a–e could be observed, as detected by a significant [M + Na+] peak, while the Diels–Alder products 1a–e were characterized by a significant [M + H+] peak. The relative stereochemistry was determined by NOESY analysis. Although compounds 1 were obtained as racemates, it is noteworthy to mention the possibility of accessing both enantiomers, through either chiral preparative HPLC or enantioselective Diels–Alder reactions,20,21 during lead optimization of identified hits.
| Entry | Nucleophilea | Product 1 | Yieldb |
|---|---|---|---|
| a 4 equiv. of nucleophile was added in the reaction. b Isolated yield after column chromatography. c Intractable mixture. | |||
| 1 | N-Methylindole | 1a | 59% |
| 2 | Indole | 1b | 56% |
| 3 | 6-Fluoroindole | 1c | 62% |
| 4 | 5-Methoxy-indole | 1d | 72% |
| 5 | 5-Fluoroindole | 1e | 60% |
| 6 | 1,3-Dimethoxybenzene | 1f | —c |
| 7 | 1H-Pyrrolo[2,3-b]pyridine | 1g | —c |
| 8 | 1H-Indazole | 1h | —c |
| 9 | Thiophene | 1i | —c |
Removal of the phthalimido protecting group of 1a–e with hydrazine and hydrochloric acid in methanol afforded the corresponding compounds with a primary amine handle, which was subjected to a subsequent round of diversification steps.§ Selected modification examples include sulfonylation with 4-(trifluoromethyl)benzene sulfonyl chloride to give 2a, TBTU-mediated acylation with cyclopropanecarboxylic acid to give 2b, urea-formation using phenyl isocyanate to give 2c, and acylation with cyclohexanecarbonyl chloride to give 2d (Fig. 3).
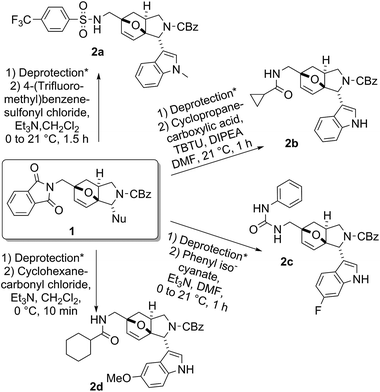 | ||
| Fig. 3 Examples of functionalized compounds 2 after the first step. * Deprotection condition: hydrazine, 2 M HCl (aq.), MeOH, 21 °C, 1 h. | ||
Cbz-deprotection of 2a–d was carried out under reducing condition using 10% Pd/C and a hydrogen atmosphere. However, except in the case of N-methyl indole substituted compound 2a, epimerization at the 3-CH-indole position with varied ratio ranging from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was observed during the deprotection for compounds 2b–d. The crude compound was simply isolated by concentration in vacuo and used directly in the next steps, since any attempts to purify it by column chromatography failed probably due to poor stability of the free amines.¶
2 was observed during the deprotection for compounds 2b–d. The crude compound was simply isolated by concentration in vacuo and used directly in the next steps, since any attempts to purify it by column chromatography failed probably due to poor stability of the free amines.¶
The secondary amino handle was then subject to another round of diversification steps. For example, the deprotected product of sulfonamide 2a was converted to the bissulfonamide 3a, alkylated compound 3b, and urea 3c. The deprotected product of amide 2b was functionalized to give sulfonylated compound 3d and acylated compound 3e through a sulfonylation reaction and a TBTU-coupling reaction, respectively, and the free amine derived from urea compound 2c was further functionalized to give sulfonylated compound 3f and amide 3g (Fig. 4).
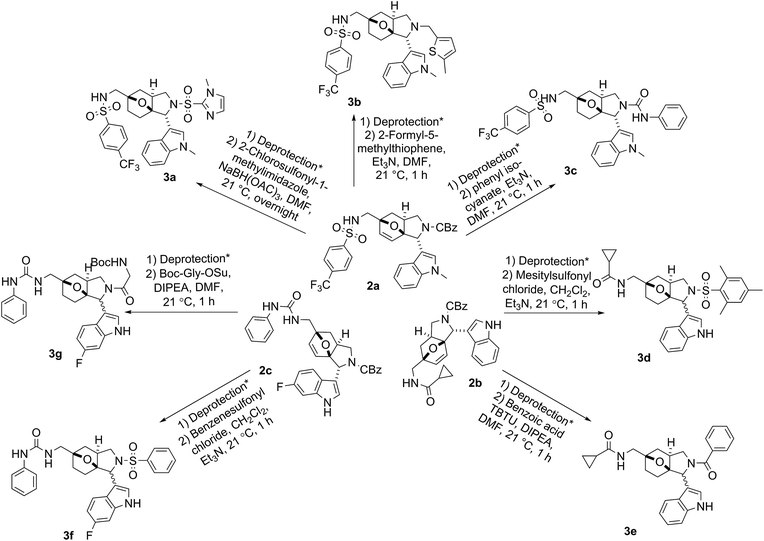 | ||
| Fig. 4 Examples of validated compounds for library synthesis. * Deprotection condition: 10% Pd/C (10 mol%), H2, MeOH, DMF, 21 °C, overnight. | ||
All of the functionalized compounds 3a–g were purified by direct preparative HPLC, which underpin the subsequent production of a screening compound library. Based on the steps of phthalimido deprotection, functionalization of primary amine, Cbz deprotection, and functionalization of the secondary amine for the synthesis of compounds with an indolyl-octahydroepoxyisoindole core, a collection of 120 compounds that resemble the structural features of compounds 3a–c have been produced as a part of a small-molecule screening library under the ELF consortium. All produced compounds and most of the enumerated compounds are compliant with Lipinski's Rule of Five in terms of clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values and MW (Fig. 5).
P values and MW (Fig. 5).
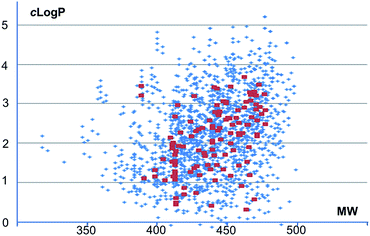 | ||
| Fig. 5 Physical chemical property analysis of the produced compounds (red dots) vs. enumerated compounds in library (blue dots). | ||
Effectively, the production focused on final compounds in N-methyl indole series since no epimerization at the 3-CH-indole position occurred during the Cbz-deprotection step. Noteworthy, the Mannich/Diels–Alder reaction was reproducible on a 0.1 mol scale with yields comparable to those shown in Table 1. To expand the library, future productions will involve additional N-substituted indoles. A systematic relative configuration assignment method could also be approached with the aim to include both diastereomers from N-unsubstituted indoles series in the library, since most of these diastereomers could be separated by preparative LCMS (C18-phase). The goal is to access new, biologically relevant chemical scaffolds that are not represented in existing screening collections.
Conclusions
An efficient protocol for the rapid assembly of indolyl-hexahydro-3a,6-epoxyisoindole via a tandem Mannich/Diels–Alder synthesis sequence has been developed in the reported study. Diversification of the indolyl-octahydroepoxyisoindole core through amino group functionalization has led to the validation of 120 compounds which will be incorporated in a small-molecule library under the ELF consortium.Acknowledgements
This research was done within the European Lead Factory and has received support from the Innovative Medicines Initiative Joint Undertaking (grant no. 115489), with financial contribution from the European Union's Seventh Framework Programme (FP7/2007–2013) and EFPIA companies' in-kind contribution. We are also grateful to the Lundbeck Foundation (R141-2013-13835), and the Technical University of Denmark for financial support. We also thank Caroline Gurcel, Luciane Adeikalam and Guillaume Ranty at Edelris for assistance in the purification of the final library compounds.Notes and references
- V. Law, C. Knox, Y. Djoumbou, T. Jewison, A. C. Guo, Y. Liu, A. Maciejewski, D. Arndt, M. Wilson, V. Neveu, A. Tang, G. Gabriel, C. Ly, S. Adamjee, Z. T. Dame, B. Han, Y. Zhou and D. S. Wishart, Nucleic Acids Res., 2014, 42, D1091–D1097 CrossRef CAS PubMed.
- P. Wu, T. E. Nielsen and M. H. Clausen, Trends Pharmacol. Sci., 2015, 36, 422–439 CrossRef CAS PubMed.
- P. Wu, T. E. Nielsen and M. H. Clausen, Drug Discovery Today, 2016, 21, 5–10 CrossRef CAS PubMed.
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed.
- C. Sherer and T. J. Snape, Eur. J. Med. Chem., 2015, 97, 552–560 CrossRef CAS PubMed.
- J.-H. Lee, T. K. Wood and J. Lee, Trends Microbiol., 2015, 23, 707–718 CrossRef CAS PubMed.
- N. Kaila, A. Huang, A. Moretto, B. Follows, K. Janz, M. Lowe, J. Thomason, T. S. Mansour, C. Hubeau, K. Page, P. Morgan, S. Fish, X. Xu, C. Williams and E. Saiah, J. Med. Chem., 2012, 55, 5088–5109 CrossRef CAS PubMed.
- J. Wang, Y. Li, Y. Yang, J. Zhang, J. Du, S. Zhang and L. Yang, RSC Adv., 2015, 5, 78278–78298 RSC.
- S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108–7149 CrossRef CAS PubMed.
- J. Besnard, P. S. Jones, A. L. Hopkins and A. D. Pannifer, Drug Discovery Today, 2015, 20, 181–186 CrossRef CAS PubMed.
- A. Karawajczyk, F. Giordanetto, J. Benningshof, D. Hamza, T. Kalliokoski, K. Pouwer, R. Morgentin, A. Nelson, G. Müller, A. Piechot and D. Tzalis, Drug Discovery Today, 2015, 20, 1310–1316 CrossRef CAS PubMed.
- P. Wu, M. Å. Petersen, R. Petersen, M. O. Rasmussen, K. Bonnet, T. E. Nielsen and M. H. Clausen, Eur. J. Org. Chem., 2015, 5633–5639 CrossRef CAS.
- R. Petersen, A. E. Cohrt, M. Å. Petersen, P. Wu, M. H. Clausen and T. E. Nielsen, Bioorg. Med. Chem., 2015, 23, 2646–2649 CrossRef CAS PubMed.
- P. Wu, M. Å. Petersen, A. E. Cohrt, R. Petersen, M. H. Clausen and T. E. Nielsen, Eur. J. Org. Chem., 2015, 2346–2350 CrossRef CAS.
- M. Å. Petersen, M. A. Mortensen, A. E. Cohrt, R. Petersen, P. Wu, N. Fleury-Brégeot, R. Morgentin, C. Lardy, T. E. Nielsen and M. H. Clausen, Bioorg. Med. Chem., 2015, 23, 2695–2698 CrossRef CAS PubMed.
- R. Pedrosa, C. Andrés and J. Nieto, J. Org. Chem., 2000, 65, 831–839 CrossRef CAS.
- C. Andrés, M. García-Valverde, J. Nieto and R. Pedrosa, J. Org. Chem., 1999, 64, 5230–5236 CrossRef.
- C. Andrés, G. Maestro, J. Nieto, R. Pedrosa, S. García-Granda and E. Pérez-Carreño, Tetrahedron Lett., 1997, 38, 1463–1466 CrossRef.
- T. A. Nevolina, T. A. Stroganova, M. V. Shevlyakov and A. V. Butin, Chem. Heterocycl. Compd., 2007, 43, 408–415 CrossRef CAS.
- M. Hatano, Y. Goto, A. Izumiseki, M. Akakura and K. Ishihara, J. Am. Chem. Soc., 2015, 137, 13472–13475 CrossRef CAS PubMed.
- N. Li, X. Liang and W. Su, RSC Adv., 2015, 5, 106234–106238 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: General methods, experimental procedures including the two deprotection steps, characterization data, 1H and 13C NMR spectra. See DOI: 10.1039/c6ra08786h |
| ‡ Authors contributed equally. |
| § The phthalimido deprotection was also tested in MeNH2, although the conversion was initially effective, it proved to be reversible, and it was difficult to perform the reaction with reproducible results. |
| ¶ An alternative approach involving the removal of the Cbz protection before phthalimido deprotection led to slow reactions in both deprotection steps. |
| This journal is © The Royal Society of Chemistry 2016 |