Supramolecular nanofibers of self-assembling peptides and DDP to inhibit cancer cell growth†
Abstract
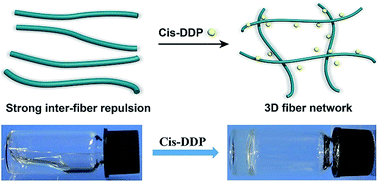
We report in this paper a molecular hydrogel formed by adding cis-dichlorodiamineplatinum(II) (DDP) to a self-assembling taxol-peptide amphiphile. Our study provides a novel self-assembling nanomedicine and hydrogel to deliver two anti-cancer drugs simultaneously.


 Please wait while we load your content...
Please wait while we load your content...