Synthesis of hierarchical ZnO/ZnCo2O4 nanosheets with mesostructures for lithium-ion anodes†
Abstract
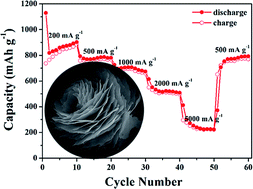
Novel bi-component-active hierarchical ZnO/ZnCo2O4 (ZZCO) nanosheets with mesostructures are successfully prepared through a convenient and practical hydrothermal route followed by a calcination process. When evaluated as anode materials for lithium-ion batteries (LIBs), the bi-component-active hierarchical ZZCO nanosheets with mesostructures exhibit highly reversible lithium storage capacity, and strong cycling stability at the 500 mA g−1 rate for 150 cycles. More significantly, the hybrid ZZCO presents an exceptionally high rate capability up to the 2 A g−1 rate.

 Please wait while we load your content...
Please wait while we load your content...