An amide functionalized task specific carbon nanotube for the sorption of tetra and hexa valent actinides: experimental and theoretical insight†
Abstract
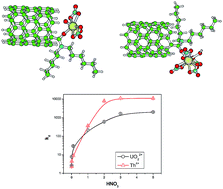
An amide functionalized multiwalled carbon nanotube (CNT-DHA) was used for efficient and selective solid phase separation of tetravalent (Th4+) and hexavalent (UO22+) actinides. Langmuir, Freundlich, Dubinin–Rodushkevich (D–R) and Tempkin isotherms were employed for understanding the sorption mechanism while various models (Lagergren first order kinetics, intra particle diffusion model and pseudo second order kinetics) were applied to understand the sorption kinetics. The sorption proceeded via monolayer coverage of CNT-DHA with capacities of 32 mg g−1 and 47 mg g−1 for UO22+ and Th4+, respectively following a Langmuir isotherm while the sorption kinetics followed a pseudo second order reaction with rate constants of 0.044 and 0.095 g mg−1 min−1 for UO22+ and Th4+, respectively. The CNT-DHA was found to have high radiolytic stability up to 1000 kGy gamma exposure. The stripping study revealed that oxalic acid can be used for almost quantitative back extraction of Th4+ and for UO22+ sodium carbonate can be effectively used. DFT calculations were performed to understand the complexation of Th4+ and UO22+ with CNT-DHA. The structural parameters of UO22+ and Th4+ ions with CNT-DHA, and the large ion–ligand interaction energy were correlated with the higher selectivity of Th4+ ions over UO22+ ions.

 Please wait while we load your content...
Please wait while we load your content...