DOI:
10.1039/C6RA06630E
(Paper)
RSC Adv., 2016,
6, 57284-57292
Air purifier devices based on adsorbents produced from valorization of different environmental hazardous materials for ammonia gas control
Received
12th March 2016
, Accepted 7th June 2016
First published on 8th June 2016
Abstract
The present study aimed to find a beneficial solution for waste recycling and disposal by converting it from an environmental load to products useful for air pollution control. This work investigated the possibility to convert cow dung, rice straw and Eichhornia crassipes (lotus plant) to activated carbons by chemical activation using phosphoric acid (H3PO4) impregnation. The prepared activated carbon samples were characterized using BET analysis for determination of the surface area and FTIR analysis was also used to give a basic understanding of the surface functional groups of the samples. The results showed that the prepared activated carbon from Eichhornia crassipes (ECAC) had the highest surface area (1200 m2 g−1) compared to activated carbons from both rice straw (RSAC, 900 m2 g−1) and cow dung (CDAC, 700 m2 g−1). The efficacy of the prepared activated carbon samples to remove ammonia gases was studied. It was noticed that the adsorption capacity of all prepared activated carbons was higher than that of the raw materials. Ammonia removal efficiency by ECAC was found to be 70% followed by RSAC 60% and finally CDAC 55%. Langmuir and Freundlich models were applied. It was recorded that Freundlich models fit the data well with R2 of 0.999. Among all prepared carbons, the best ECAC was selected to develop a new air filter device. The performance of the new filter was examined in the field for removing ammonia gases from indoor air of a cow barn. It was found that the filter is effective to remove ammonia gases (70%) from the polluted area during the experimental period (15 days); the filter adsorption capacity was slightly decreased at the end of the experiment but it is still effective. Hence we can say that this new filter can act as an economic product used to remove air pollutants as well as a way to overcome the problem of different environmental pollutants and convert these wastes into useful products for the environment.
1. Introduction
Environmental wastes have become a great problem for the environment. The different ways to get rid of these wastes have not lead to desired results. These processes themselves may be a source for the production of many pollutants more dangerous to the environment. On the other hand, the high cost of these processes must be taken into consideration. For all of these reasons, there was a general orientation for recycling the various environmental wastes and other materials which cause a lot of environmental problems. One of the major environmental wastes is rice straw, which represents a significant influence on the environment by its burning.1,2 Burning of straw could be the main source for hazard materials and other gaseous pollutants and lead to the black cloud problem.3 Animal dung is also considered an environmental waste. It is used as a source of energy in the primitive countryside when burned for cooking or heating thus leading to the emission of different air pollutants such as volatile organic compounds.4,5 We can also mention the widespread growth of Eichhornia crassipes as one source that has caused many environmental and economic problems.6 Eichhornia crassipes is an aquatic plant that grows in rivers and causes many problems such as the destruction of biodiversity,7 oxygen depletion and reduced water quality and clogging of watercourses that hinder agriculture, fisheries, entertainment and hydropower.8 Without finding appropriate ways of re-utilization and treatment of these different environmental wastes (cow dung and rice straw) as well as Eichhornia crassipes, they may cause massive environmental and economic problems. These wastes, as they are composed of cellulose and lignin, can be considered as raw materials for activated carbon manufacturing.9 Thus we can use these activated carbons for water and air treatments from different environmental pollutants. Therefore, we can valorize these wastes by different ways to get useful and economic products from available and cheap raw materials.
The first objective of this study is to recycle the cow dung, rice straw as well as Eichhornia crassipes (aquatic plant) producing different activated carbons via chemical activation by impregnated phosphoric acid (H3PO4). The second objective is to examine the performance of different prepared activated carbons for removing ammonia gases at the laboratory scale. Finally, development of an economic filter using the best prepared activated carbon as an adsorbent and the performance of this new filter for ammonia removal from a cow barn were investigated. The present study chose the application of the AC filters for ammonia removal from air in a cow barn due to the fact that ammonia is a common by-product of animal waste due to the inefficient conversion of feed nitrogen into animal products. Nitrogen that is not metabolized into animal protein (i.e., milk, meat, or eggs) is excreted in the urine and feces where further microbial decomposition of animal wastes is the main source of ammonia volatilization.
Elevated levels of ammonia can have a negative impact on animal health and production. Moreover, reduced final body weights have been observed in poultry produced in houses with indoor ammonia levels of approximately 25 parts per million (ppm) or higher during brooding.10 Ammonia can also have a negative impact on human health. Exposure to even low levels of ammonia can irritate the lungs and eyes. The Occupational Safety and Health Administration (OSHA) have established a permissible worker exposure limit for ammonia of 50 ppm over an eight-hour period. The American Conference of Governmental Industrial Hygienists (ACGIH) has recommended a short-term (15 minute) ammonia exposure limit of 35 ppm.
2. Materials and methods
2.1. Materials
Rice straw and cow dung wastes were collected from the local market. Eichhornia crassipes samples have been collected from the River Nile (Cairo-Egypt). Samples have been washed with boiled water to remove dust and other impurities, air-dried in sunlight until all the moisture was evaporated, cut into short pieces and crushed into coarse grains. All the chemicals used were analytical grade reagents supplied by Sigma Aldrich.
2.2. Preparation of activated carbon
In this study, the activated carbon was produced through the chemical activation process. 50 g of the dried raw materials were treated with 50% analytical grade phosphoric acid (100 mL) for 24 h and then separated by decantation. The impregnated raw material was transported to a stainless steel tubular furnace system. The soaked sample was firstly heated to 170 °C at a rate of 10 °C min−1 and kept at this temperature for 30 min to remove water. The activation temperature was then raised slowly until it reached 500 °C with a heating rate of 10 °C min−1 under atmospheric nitrogen for 2 h. The produced carbon was cooled and then it was subjected to thorough washing with hot water until reaching neutral pH. Then the samples were dried in an oven at 110 °C overnight. For our study, the acidic wastes obtained in the synthetic protocol have been collected in containers, and were disposed of in collaboration with other laboratories in the National Research Centre (Egypt). However, if we applied this study at a large scale, we must collaborate with other organizations which are interested in the protection of the environment in Egypt to devise the best organization for the disposal of all hazardous wastes.
2.3. Characterization of prepared activated carbon
(a) Specific surface area and pore volume. The surface area of the prepared activated carbon samples was determined using nitrogen as the sorbate at 77 K in a static volumetric apparatus (Quantachrome NOVA Automated Gas Sorption System). Specific total surface areas were calculated using the BET equation (eqn (1)):| |
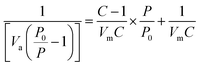 | (1) |
where, P is the partial vapour pressure of the adsorbate gas in equilibrium with the surface at 77.4 K (b.p. of liquid nitrogen), in pascals, P0 is the saturated pressure of the adsorbate gas, in pascals, Va is the volume of the gas adsorbed at standard temperature and pressure (STP) [273.15 K and atmospheric pressure (1.013 × 105 Pa)], in milliliters, Vm is the volume of the gas adsorbed to produce an apparent monolayer on the sample surface, in milliliters, and C is the dimensionless constant that is related to the enthalpy of adsorption of the adsorbate gas on the powder sample.
(b) Fourier transform infrared spectroscopy (FTIR) analysis. A known mass (1 mg) of the activated carbon sample was ground and milled with 100 mg of KBr to form a fine powder. This powder was then compressed into a thin pellet under 7 tons for 5 minutes. The sample was analyzed using a spectrometer and the spectrum was recorded in a spectral range.
2.4. Ammonia gas adsorption by prepared activated carbon samples
Fig. 1 shows the experimental study used to examine the adsorption capacity of the prepared activated carbon samples towards ammonia gas removal and compared it to that of the original raw materials. The adsorbent (1 g) of the prepared activated carbon or raw material was introduced into the angled tube of the batch glass reactor (0.5 dm3). Then, a known volume of liquid ammonia was introduced into a Petri-dish in each adsorption reactor at 25 °C and 101 kPa. After the complete evaporation of ammonia, the angled tube was turned to introduce the adsorbent into the adsorption chamber. The adsorption chambers were stirred until the equilibrium was reached. Prior to the experiment, different volumes of ammonia solution were evaporated into the empty reactor under the same experimental conditions to determine the corresponding initial ammonia concentration in the system. The initial ammonia concentrations were in the range of 950–2500 μg m−3. The effect of contact time on the percentage removal of ammonia gas by prepared AC was investigated at a fixed initial ammonia concentration of 1466 μg m−3. Finally, gas samples were collected from each reactor by withdrawing air with a constant flow rate of 1 L min−1 for 15 min through a bubbler containing 25 mL of slightly acidified water as the NH3 absorbent solution. The concentration of ammonia was determined by a colorimetric method using Nessler’s reagent.11 Ammonia samples were measured using an ultraviolet/visible (UV/VIS) spectrophotometer (NOVASPEC 4049 Spectrophotometer LKB BIOCHROM) at 460 nm.
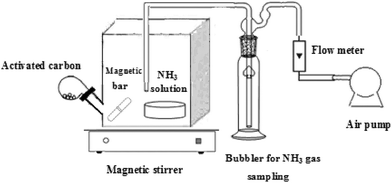 |
| | Fig. 1 Schematic diagram of the experimental set-up used in ammonia gas adsorption by the prepared activated carbons. | |
2.5. Adsorption modeling
Among the wide variety of available theoretical models and empirical fitting functions used in this field, the ammonia isotherms were evaluated according to the two parameter models of Langmuir and Freundlich. The adsorption model constants, the values of which express the surface properties and affinity of the adsorbent, can be used to compare the adsorption capacity of adsorbents for different adsorbates.
Langmuir equation is suitable for monolayer adsorption onto a surface with a fixed number of identical sites and is given by eqn (2):
| |
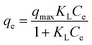 | (2) |
where
qe is the amount of adsorbate fixed on the adsorbent at equilibrium (μg g
−1),
Ce is the equilibrium concentration of the adsorbate (μg mL
−1), and
qmax and
KL are the Langmuir constants, representing the maximum adsorption capacity for the solid phase loading and the energy constant related to the heat of adsorption, respectively. A high
KL value reveals a strong interaction between the pollutant and the surface of the adsorbent. The Langmuir parameters were evaluated by linearization of
Ce/
qe versus Ce.
The Freundlich model is an empirical equation based on adsorption on a heterogeneous surface. It is given by eqn (3):
where
KF is the Freundlich constant for a heterogeneous adsorbent, and
n is related to the magnitude of the adsorption driving force and to the adsorbent site energy distribution. The value of
n indicates favorable adsorption when 1 <
n < 10; the more favorable, the lower its value within this range. The Freundlich parameters were determined by linearization of log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus
qe versus log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce.
2.6. Application of the new activated carbon filter in the field for indoor ammonia gas control
The present study is directed to provide an economic activated carbon air purifier that can be conveniently installed with a small volume (1454.2 cm3) and has high gas contaminant removal efficiency. The new filter made from the AC sample (15 g) was prepared from Eichhornia crassipes aquatic Nile plant. The new activated carbon filter has been fabricated and constructed in Egypt at the National Research Center. Fig. 2a shows the schematic structure of the new filter. The device is cylindrical in shape with a height of 14 cm; the body frame is made from PVC since it is a heating and electrical insulator material. The top and bottom covers of the device are stainless steel mesh and there are a number of blades on the two wall sides for device temperature control to avoid overheating of the device during operation where high temperature is not recommended for the adsorption process (exothermic process). A small fan is located in the upper portion of the side cover of the filter for distributing the treated air. A stream flow of air passes from the bottom of the filter by an air pump. The central section of the present filter is a stainless steel cylindrical mesh (Fig. 2b) with a diameter three times smaller than that of the principal frame (11.5 cm) thus this design allows air passage through the filter for treatment. Fig. 2b shows the activated carbon filter material is steady over the central cylinder. The activated carbon sample was used in its granular form to minimize the pressure drop in the adsorption filter and decrease the energy consumption. The granular AC is distributed in a housing sac of the synthetic fiber without any adhesive materials. The synthetic housing is divided into several chambers and wrapped with a sponge that helps to maintain efficiency and extends the life of the filter and makes it easy to handle. Fig. 2c illustrates the side portion of filter including a number of perforated pipes where there is one in each chamber; the pipes are hooked up together via the selector valve and connect with the air pump, which is operable to produce a flow of air. The pipes also provide additional strength to the filter housing.
 |
| | Fig. 2 Schematic structural view of the new filter (a), central view of the filter based activated carbon (b), and inside view of the filter and the perforated pipes (c). | |
The performance of this new filter was investigated for NH3 gas removal from indoor air of a small cow barn (3 m × 4 m, contain 8 cows) located in the Menofia countryside area. Sampling in the barn was located at a moderate height (1.5 m) in the middle area. The indoor air samples from the barn area were firstly collected over 7 days before using the filter for determining the initial indoor ammonia concentrations in the cow barn. Samples were collected by withdrawing air with a constant flow rate overnight (1 L min−1, for 12 hours) through a bubbler with a volume of 100 mL containing the NH3 gas absorbent solution. The indoor air samples had been collected before and after using the new air purifier filter. The new filter has been used to evaluate ammonia odor control for 7 consecutive days. According to this test, the obtained data could give primary evidence for how this new filter is effective for use in an indoor air control field.
3. Results and discussion
3.1. Characterization of the studied activated carbons
Table 1 shows that the BET surface area of the ECAC was about 1200 m2 g−1 which was the highest value among all prepared AC samples followed by RSAC (SBET, 900 m2 g−1), then CDAC sample, which has the lowest surface area (SBET, 700 m2 g−1). The same values of the AC surface area from different agriculture wastes by chemical activation were observed by several researchers.12,13 The different specific surface areas of the prepared AC samples are related to their porosity, which is primarily attributed to the properties of the precursors. The results showed that the ECAC sample exhibited much more microporosity. It has a total mesoporous volume of 0.58 cm3 g−1 and a microporous volume of 0.21 cm3 g−1. This observation coincided with Lei et al.14 who found mesoporous activated carbon prepared from Jerusalem artichoke stem precursor by ZnCl2 activation. Moreover, it was cited that the surface area values of the prepared AC were in the same range of that of several commercial ACs (S23 and F22).15
Table 1 Main characteristics of the prepared activated carbon samples
| Activated carbon sample |
ECAC |
RSAC |
CDAC |
| Origin |
Eichhornia crassipes |
Rice straw |
Cow dung |
| Specific surface (m2 g−1) |
1200 |
900 |
700 |
| Microporous volume (cm3 g−1) |
0.21 |
0.11 |
0.04 |
| Mesoporous volume (cm3 g−1) |
0.58 |
0.35 |
0.20 |
| Pore size diameter (Å) |
20 |
19 |
17 |
The FT-IR spectroscopic study of the prepared activated carbons and the raw materials is shown in Fig. 3. The results of FT-IR analysis indicate that only minor differences between the samples could be established. However, a shift in the band absorbance and changes in intensities between the starting raw materials and prepared AC samples showed that chemical transformation had taken place during the chemical activation process. All activated carbon samples displayed a similar adsorption band with various intensities. The spectra of the adsorbent display a number of absorption peaks, indicating that many functional groups are present in the different samples. FTIR analysis showed the presence of three major absorption bands at 2900–3500 cm−1, 1300–1750 cm−1 and 1000–1250 cm−1. The band in the range of 3200–3650 cm−1 is due to the absorption of water molecules as a result of an O–H stretching mode of hydroxyl groups present in all samples (prepared activated carbon samples and the original raw material) but with different signals. This band was also attributed to the hydrogen-bonded OH group of alcohols and phenols.16 The prepared AC exhibits carbonyl functional groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1740 cm−1).17 Moreover, the band at 1500 cm−1 may be attributed to the aromatic carbon–carbon stretching vibration. The bands observed in the range of 1000 to 1260 cm−1 indicated a C–O single bond of the carboxylic acids and alcohols.18 These results clearly show that the functional groups obtained during the activation process, including carboxylic and hydroxyl groups contribute to the adsorption mechanism of the activated carbon.19 The formation of acidic groups at the carbon surface prepared by chemical activation with H3PO4 is in agreement with other citations.20–23
O at 1740 cm−1).17 Moreover, the band at 1500 cm−1 may be attributed to the aromatic carbon–carbon stretching vibration. The bands observed in the range of 1000 to 1260 cm−1 indicated a C–O single bond of the carboxylic acids and alcohols.18 These results clearly show that the functional groups obtained during the activation process, including carboxylic and hydroxyl groups contribute to the adsorption mechanism of the activated carbon.19 The formation of acidic groups at the carbon surface prepared by chemical activation with H3PO4 is in agreement with other citations.20–23
 |
| | Fig. 3 Comparison of FTIR spectra between the prepared activated carbons; ECAC (a), RCAC (b) and CDAC (c) and that of the original raw materials. | |
When concentrated H3PO4 is used at high temperature, it appears to function both as an acid catalyst to promote bond cleavage reactions and formation of new bonds and to combine with organic species to form phosphate and polyphosphate bridges. Various AC surface acidic functional groups (oxygen- and/or phosphorus-containing groups) are developed through the surface oxidation by H3PO4 as well as attachment of different oxygen/phosphorous groups to the surface.24
3.2. Kinetic adsorption of ammonia gas onto prepared activated carbons
Fig. 4 represents the experimental results obtained for ammonia adsorbed as a function of time onto the surface of ECAC, RSAC and CDAC samples. The results showed that the time taken to reach equilibrium was about 72 hours for all the samples. Fig. 4 shows that for all AC samples the ammonia gas adsorption increased rapidly at the first time of contact (rapid adsorption phase) due to the presence of free adsorption sites onto the activated carbon at the beginning of the experiment and then the adsorption gradually decreased (steady state phase) to reach equilibrium. Rashidi et al.25 reported that the rapid rate of adsorption could be related to the adsorbent surface area which interacts with carbon dioxide gas. Moreover, the rate of adsorption is found to decrease as a result of the reduction of active sites over time, which may slow down the adsorption process. Li et al.26 suggested that the rapid adsorption at the beginning of the process is due to the external surface of the adsorbent then, by the time the slower adsorption was observed, it was due to a slower internal diffusion process. The adsorption kinetic study investigated that the ammonia gas adsorption was faster with an ECAC than those with RSAC and CDAC samples. Fig. 5 shows that at the end of the adsorption run, approximately 70% of the pollutant was adsorbed by ECAC while nearly 60% had been adsorbed by both RSAC and CDAC samples. The possible explanation for the slower adsorption onto RSAC and CDAC could be due to the different BET surface areas and the lower mesoporous and microporous volumes. Ro et al.27 reported that phosphoric acid activated biochars show a high capacity of ammonia adsorption.
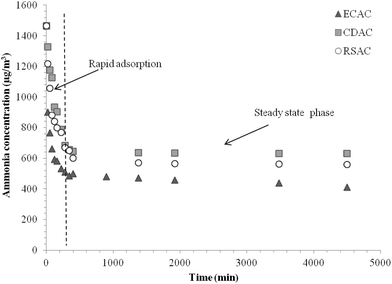 |
| | Fig. 4 Kinetic adsorption of ammonia gas by the prepared activated carbons. | |
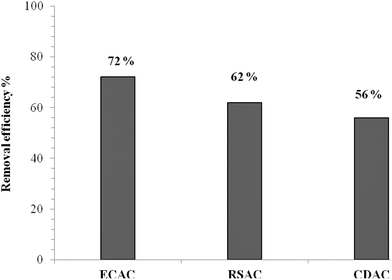 |
| | Fig. 5 Ammonia removal efficiency using three prepared activated carbon samples. | |
3.3. Screening of the adsorption performance of three prepared activated carbons of various origins
A comparison between the adsorption performances of the prepared AC samples and that of the original raw materials is represented in Fig. 6. The results showed that whatever the adsorbent used, all the prepared activated carbons revealed the strongest adsorption capacity of ammonia gas as compared to the original raw materials. This result may be explained by the high BET surface areas of ACs28 and/or the surface functional groups on the prepared AC samples due to the activation process.29 The comparison of three AC samples showed that ECAC is the best adsorbent followed by RSAC and then CDAC with a range of investigated ammonia concentrations. ECAC (SBET, 1200 m2 g−1) presents the highest specific surface, while CDAC has the lowest one (700 m2 g−1). FTIR analysis showed the acidic characteristics of AC chemical surfaces due to the impregnation of H3PO4 during the AC sample preparation. Thus, the ammonia gas (basic gas) adsorption capacity increased via a Bronsted acid–base process.30 Similarly, Huang et al.26 reported that the acidic functional groups on the adsorbent surface aide a great deal in improving the adsorption of ammonia gas. As a way of comparison, Table 3 lists the amount of adsorbed ammonia gas onto prepared activated carbon samples (present work) and that from literature references using different activated carbon adsorbents. As can be seen, adsorption capacities of prepared activated carbon samples toward ammonia gas are of the same order of those observed values using other activated carbon adsorbents. In general, gaseous ammonia sorption capacities of commercial activated carbon range from 0.6 to 6 mg g−1 (Table 3).
 |
| | Fig. 6 Comparison between adsorption isotherms of three prepared activated carbons; ECAC (a), RSAC (b), CDAC (c) and that of raw materials. | |
Table 2 Parameters of Langmuir and Freundlich models for ammonia adsorption onto three AC samples at 25 °C
| AC samples |
Langmuir model (version 1) |
Freundlich model |
| qmax (μg g−1) |
KL (m3 μg−1) |
R2 |
n |
KF (μg g−1) (m3 μg−1)1/n |
R2 |
| ECAC |
2500 |
0.0005 |
0.86 |
1.3 |
0.32 |
0.99 |
| RSAC |
1111 |
0.0008 |
0.78 |
1.6 |
0.03 |
0.98 |
| CDAC |
1111 |
0.0007 |
0.66 |
1.7 |
0.01 |
0.98 |
Table 3 Comparison of adsorption capacities towards ammonia gas between the prepared activated carbon samples and different adsorbents reported in the literaturea
| Adsorbent |
Specific surface area (m2 g−1) |
Adsorption capacity (mg g−1) |
References |
| n/a not available. |
| ECAC |
1200 |
2.5 |
Present work |
| RSAC |
900 |
1.1 |
Present work |
| CDAC |
700 |
1.1 |
Present work |
| GAC (Cherng Tay Corporation) |
1250 |
0.6 |
31 |
| MA2 (spherical resin-based AC, supplied by MAST Carbon International, UK) |
1550 |
4.9 |
30 |
| ACF-10 (Activated carbon fibers) |
760 |
0.97 |
32 |
| Chemsorb 1425 (coconut-shell, GAC) |
n/a |
2.4 |
33 |
| BPL carbon (Calgon corporation) |
1033 |
0.8 |
34 |
| GAC (fixed bed) |
n/a |
1.8 |
35 |
| Merck-AC |
451 |
5.5 |
36 |
3.4. Model application for ammonia gas adsorption
For a comparison of ammonia isotherm results in a quantitative manner, the isotherms were evaluated according to two major parameter models, Langmuir and Freundlich and the constants obtained according to these two models are listed in Table 2. The correlation coefficient squared (R2) for both models at 25 °C suggested that the empirical Freundlich equation was more convenient (R2 = ∼0.99) than that of Langmuir (R2 = ∼0.8) for describing the adsorption of ammonia gas molecules onto all prepared AC samples.
Fig. 7 shows the experimental equilibrium data for ammonia adsorption isotherms and the predicted theoretical Freundlich model. These results showed that the ammonia adsorption capacity of ECAC was higher than that of the two other AC samples. As mentioned before, this higher performance of ECAC could be due to the much higher BET surface area (1200 m2 g−1) and its highly mesoporous structure. It was noticed that ammonia gas adsorption isotherms onto all prepared AC samples were type II. The later isotherm indicated the formation of ammonia multilayer adsorption coinciding with additional interactions between ammonia gas molecules. The possibility of the formation of these layers is generally in agreement with the mesoporous properties of the prepared AC samples.37 The obtained results in Fig. 6 confirm that the Freundlich model fit well with the experimental data of ammonia adsorption by ECAC, RSAC and CDAC. The constant parameters of the Langmuir and Freundlich models are represented in Table 2. The Freundlich model constants indicated that the ECAC sample displayed the highest KF (0.32) value compared to those of the RSAC and CDAC samples (0.03 and 0.01, respectively). Thus, this result confirmed that the adsorption capacity of ECAC is higher than that of RSAC and CDAC. The n value also suggested that the prepared AC samples have high adsorption intensity. Similarly, it was reported in the literature that the values n > 1 represent favorable phenol adsorption by AC derived from sugarcane bagasse.38
 |
| | Fig. 7 Experimental equilibrium data of the ammonia adsorption isotherm and the predicted theoretical Freundlich model. | |
3.5. Field application of the AC based new filter for ammonia gas control from indoor air
The new air purifier filter prepared from ECAC (the best AC sample from laboratory experimental studies) was examined for ammonia gas removal from indoor air in a small cow barn. Fig. 8 shows that the initial indoor ammonia concentrations (prior to filter application) in the air samples collected from the cow barn over 7 days ranged from 1213 to 1356 μg m−3. The ammonia concentration in the cow barn indoor air sharply decreased to 433 μg m−3 after 12 h in the first day of filter application. On the second and third day of filter application, the ammonia gas concentrations were found to be 513 μg m−3 and 600 μg m−3, respectively. It was found that the ammonia gas molecules were still significantly removed from the indoor air of the cow barn by consecutive filter reuse and reached 736 μg m−3 in the last application day. The results in Fig. 9 reveal the filter removal efficiency by studying the difference in ammonia concentrations before and after using the filter. The results showed that approximately 70% of the ammonia was removed on the first day of filter application. The removal percentage of ammonia gas by the prepared activated carbon ECAC was approximately similar to that obtained from literature citations using different commercial activated carbons.39
 |
| | Fig. 8 Ammonia concentration in an animal house (cow barn) before and after using the new AC based filter. | |
 |
| | Fig. 9 Ammonia removal efficiency over the treatment period of the animal house by the new filter. | |
The removal efficiency was then slightly decreased to more than 50% on the second, third and fourth day. A further decrease in ammonia removal had reached 40% on the 7th day. This decrease in ammonia removal efficiency could be related to the fact that some of the AC adsorption sites were saturated and thus a decrease in the adsorption capacity of the AC was observed during the application period.40 At the end of the filter application, the removal percentage indicated that it is still capable of removing more and more ammonia gas. Consequently, the new filter performance on the field-scale showed that the filter is effective as an indoor air adsorbent treatment system.
4. Conclusions
This research work proves the effectiveness of activated carbons prepared from rice straw, cow dung and Eichhornia crassipes (aquatic plant) by using a chemical activation process. The prepared carbons ECAC, RSAC and CDAC have a BET surface area (SBET) of 1200 m2 g−1, 900 m2 g−1 and 700 m2 g−1, respectively. For economic costs, it was assumed that there is a considerable differential cost between the production costs of activated carbon from different raw materials by chemical activation and the selling price for the commercial activated carbon. Moreover, the economic significance of large scale production is also important. The low cost and high availability of raw materials would be minimized the manufacture costs. On the other hand, this technique can be considered the best solution for disposal of materials and wastes that cause environmental inconvenience. In terms of their chemical properties, the prepared AC samples were acidic in nature and the FTIR analysis confirmed the presence of acidic functional groups such as carbonyl groups, alcohols, phenols and carboxylic acid groups. The results showed that acidic activated carbons prepared from rice straw, cow dung and an aquatic plant (Eichhornia crassipes) by chemical activation via H3PO4 impregnation were suitable for use as an adsorbent for basic gas removal. The laboratory experiment kinetic study showed that ammonia gas adsorption was faster with an ECAC than with RSAC and CDAC samples. In addition, ECAC showed the highest ammonia adsorption capacity (70% of pollutants being adsorbed) while nearly 60% had been adsorbed by each of the RSAC and CDAC samples. The results preliminarily proved that the new filter based on cheap activated carbon from the available raw material can exhibit an excellent performance for ammonia gas removal. The new filter achieved the removal of ammonia gas of more than 70% on the first application day from the indoor air of the cow barn. Moreover, the new filter can be reused and rest effective after the last application day (40%). The new filter operating costs are usually considerably less than that by using the commercial activated carbon. Consequently, the new filter can be successfully used in the field for indoor air pollutant control.
Acknowledgements
The authors are grateful to National Research Centre, for providing laboratory facilities towards successful completion of this work.
References
- Z. Liu, A. Xu and T. Zhao, Energy Power Eng., 2011, 3, 325–331 CrossRef CAS.
- C. Chang, C. Liu and P. Tseng, Aerosol Air Qual. Res., 2013, 13, 474–487 CAS.
- M. Moussa and A. Abdelkhalek, J. Appl. Sci. Res., 2007, 3, 147–154 Search PubMed.
- O. Kurmi, K. Lam and G. Ayres, Eur. Respir. J., 2012, 40, 239–254 CrossRef PubMed.
- M. Ezzati and D. Kammen, Lancet, 2001, 358, 619–624 CrossRef CAS.
- T. Téllez, E. López, G. Granado, E. Pérez, R. López and J. Guzmán, Aquatic Invasions, 2008, 3, 42–53 CrossRef.
- P. Aloo, W. Ojwang, R. Omondi, J. Njiru and D. Oyug, Biodiversity Journal, 2013, 4, 471–482 Search PubMed.
- M. Nasution, S. Awal and D. Permana, Int. J. Environ. Sci. Dev., 2016, 7, 630–633 CrossRef.
- M. Soleimani and T. Kaghazchi, Adv. Chem. Eng. Res., 2014, 3, 34–41 Search PubMed.
- F. N. Reece, B. D. Lott and J. W. Deaton, Poult. Sci., 1980, 59, 486–488 CrossRef CAS.
- M. Harrison and R. Perry, Handbook of Air Pollution Analysis, Chapman and Hall, London, New York, 2nd edn, 1986 Search PubMed.
- R. Hesas, A. Niya, W. Daud and J. Sahu, BioResources, 2013, 8, 2950–2966 Search PubMed.
- M. Aji, B. Gutti and B. Highina, Columban J. Life Sci., 2015, 17, 18–24 Search PubMed.
- Y. Lei, C. Tu and Y. Luo, Front. Environ. Sci. Eng., 2015, 9, 206–215 CrossRef.
- E. F. Mohamed, C. Andriantsiferana, A. M. Wilhelm and H. Delmas, Environ. Tech., 2011, 32, 1325–1336 CrossRef CAS PubMed.
- K. Venkatachalam, P. Visuvamithiran, B. Sundaravel, M. Palanichamy and V. Murugesan, Chin. J. Catal., 2012, 33, 478–486 CrossRef CAS.
- R. Rajbhandari, L. Shrestha, B. Pokharel and R. Pradhananga, J. Nanosci. Nanotechnol., 2013, 13, 1–11 CrossRef.
- H. Yaoxin, D. Xueliang, N. Jiangpu, J. Wanqin, R. Xiaoming, X. Nanping and Y. Moo, Chem. Commun., 2011, 47, 737–739 RSC.
- X. Zheng, X. Jin, W. Zhenzhen and L. Weihao, Desalin. Water Treat., 2015, 56, 2543–2550 CrossRef.
- R. Bansode, J. Losso, W. Marshall, R. Rao and R. Portier, Bioresour. Technol., 2003, 90, 175–184 CrossRef CAS PubMed.
- B. Corral, M. Marin, C. Gonzalez, V. Serrano and M. Garcia, Appl. Surf. Sci., 2006, 252, 5961–5966 CrossRef.
- M. Rahman, M. Adil, A. Yusof, Y. Kamaruzzaman and R. Ansary, Materials, 2014, 7, 3634–3650 CrossRef CAS.
- A. Yuso, B. Rubio and M. Izquierdo, Fuel Process. Technol., 2014, 119, 74–80 CrossRef.
- S. A. Dastgheib and D. A. Rockstraw, Carbon, 2001, 39, 1849–1855 CrossRef CAS.
- N. Rashidi, S. Yusup and H. Lam, Chem. Eng. Trans., 2013, 35, 361–366 Search PubMed.
- C. Huang, H. Li and C. Chen, J. Hazard. Mater., 2008, 311, 311–314 Search PubMed.
- K. Ro, I. Lima, G. Reddy, M. Jackson and B. Gao, Agriculture, 2015, 5, 991–1002 CrossRef.
- E. Mohamed, C. Andriantsiferana, A. Wilhelm and H. Delmas, Environ. Technol., 2011, 32, 1325–1336 CrossRef CAS PubMed.
- Z. Lu, J. Hines, D. Rozewicz and M. Hines, Am. J. Anal. Chem., 2013, 4, 776–780 CrossRef CAS.
- M. Goncalves, L. S. Garcia, E. O. Jardim, J. S. Albero and F. R. Reinoso, Environ. Sci. Technol., 2011, 45, 10605–10610 CrossRef CAS PubMed.
- Y. C. Chung, Y. Y. Lin and C. P. Tseng, Bioresour. Technol., 2005, 96, 1812–1820 CrossRef CAS PubMed.
- C. L. Mangun, R. D. Braatz, J. Economy and A. J. Hall, Ind. Eng. Chem. Res., 1999, 38, 3499–3504 CrossRef CAS.
- O. Monje, J. M. Surma and M. N. Johnsey, 4th International Conference on Environmental Systems ICES, Tucson, Arizona, 13–17 July 2014 Search PubMed.
- T. J. Bandosz and C. Petit, J. Colloid Interface Sci., 2009, 338, 329–345 CrossRef CAS PubMed.
- C. C. Rodrigues, D. J. de Moraes, S. W. da Nóbrega and M. G. Barboza, Bioresour. Technol., 2007, 98, 886–891 CrossRef CAS PubMed.
- J. Helminen, J. Helenius, E. Paatero and I. Turunen, AIChE J., 2000, 46, 1541–1555 CrossRef CAS.
- C. J. Lebigue, C. Andriantsiferana, K. N’Guessan, C. Ayral, E. Mohamed, A. M. Wilhelm, H. Delmas, L. Le Coq, C. Gerente, K. M. Smith, S. Pullket, G. D. Fowler and N. J. Graham, J. Environ. Manage., 2010, 91, 2432–2439 CrossRef PubMed.
- K. P. Singh, A. Malik, S. Sinha and P. Ojha, J. Hazard. Mater., 2008, 150, 626–641 CrossRef CAS PubMed.
- I. Tsutomu, A. Takashi, K. Kuniaki and O. Kikuo, J. Health Sci., 2004, 50, 148–153 CrossRef.
- E. F. Mohamed, M. A. El-Hashemy, N. M. Abdel-Latif and W. H. Shetaya, J. Air Waste Manage. Assoc., 2015, 65, 1413–1420 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 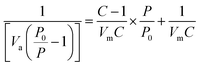

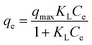
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus log
qe versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce.
Ce.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1740 cm−1).17 Moreover, the band at 1500 cm−1 may be attributed to the aromatic carbon–carbon stretching vibration. The bands observed in the range of 1000 to 1260 cm−1 indicated a C–O single bond of the carboxylic acids and alcohols.18 These results clearly show that the functional groups obtained during the activation process, including carboxylic and hydroxyl groups contribute to the adsorption mechanism of the activated carbon.19 The formation of acidic groups at the carbon surface prepared by chemical activation with H3PO4 is in agreement with other citations.20–23
O at 1740 cm−1).17 Moreover, the band at 1500 cm−1 may be attributed to the aromatic carbon–carbon stretching vibration. The bands observed in the range of 1000 to 1260 cm−1 indicated a C–O single bond of the carboxylic acids and alcohols.18 These results clearly show that the functional groups obtained during the activation process, including carboxylic and hydroxyl groups contribute to the adsorption mechanism of the activated carbon.19 The formation of acidic groups at the carbon surface prepared by chemical activation with H3PO4 is in agreement with other citations.20–23






