HDACI regulates the PI3K/Akt signaling pathway to reverse MCF-7/PTX resistance by inhibiting SET
Weipeng Zhangab,
Xiaowei Zhenga,
Ti Menga,
Haisheng Youa,
Yalin Donga,
Jianfeng Xing*c and
Siying Chen*a
aDepartment of Pharmacy, The First Affiliated Hospital of Xi'an Jiaotong University, No. 277 of Yanta West Road, Xi'an, Shaanxi 710061, PR China. E-mail: ychen0326@163.com; Fax: +86-29-85323240; Tel: +86-29-85323243
bDepartment of Pharmacy, The Eighth Hospital of Xi'an, Xi'an, Shaanxi 710061, PR China
cSchool of Pharmacy, Xi'an Jiaotong University, No. 76 of Yanta West Road, Xi'an, Shaanxi 710061, PR China. E-mail: xajdxjf@mail.xjtu.edu.cn; Fax: +86-29-82655130; Tel: +86-29-82655130
First published on 9th May 2016
Abstract
The occurrence of chemoresistance greatly restricts the efficacy of antitumor drugs, and so novel agents are urgently needed to abrogate resistant phenotypes. In this study we investigated the role of patient SE translation (SET) in the human breast cancer paclitaxel-resistant cell line (MCF-7/PTX) and the underlying reversal mechanism of FA18, a new synthetic histone deacetylase inhibitor. The expressions of SET, protein phosphatase 2A (PP2A), and factors related to the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway and downstream mitochondrial apoptosis pathways were determined in MCF-7/PTX cells. In order to explore the role of SET in inducing drug resistance, a SET antagonist OP449 was used to down-regulate SET in MCF-7/PTX cells, and the cell viability, cell apoptosis, and pathway-related factors were assessed. Furthermore, the reversal effects of FA18 and its underlying mechanisms were investigated in vitro and in vivo. We found that SET and the PI3K/Akt signaling pathway were activated in MCF-7/PTX cells. The down-regulation of SET by OP449 significantly sensitized MCF-7/PTX cells to paclitaxel (PTX) and induced cell apoptosis. In addition, SET-induced PTX resistance was associated with activation of the PI3K/Akt signaling pathway and inhibition of mitochondrial apoptosis pathways. FA18 significantly reduced the mRNA and protein levels of SET in MCF-7/PTX cells. Furthermore, FA18 significantly inhibited SET-mediated resistance by attenuating the PI3K/Akt signaling pathway and activating mitochondrial apoptosis pathways. Additionally, FA18 significantly reduced the SET level via the PI3K/Akt signaling pathway both in vitro and in vivo. These results suggest that the overexpression of SET is associated with drug resistance in MCF-7/PTX cells. FA18 reversed this drug resistance, possibly via suppression of the SET/PI3K/Akt signaling pathway.
Introduction
Breast cancer is a malignant tumor in women whose prevalence has increased steadily over the past few decades. Breast cancer seriously impacts the physical and mental health of women worldwide, and it is often life-threatening.1 Chemotherapy plays a major role in cancer therapy alongside operative treatment, and it has definite therapeutic effects. Paclitaxel (PTX) exhibits significant antitumor activity, and it is widely used as a primary treatment for breast cancer. However, a main cause contributing to the failure of chemotherapy is the development of drug resistance in breast cancer patients.2 Potential mechanisms underlying the resistance to PTX have been reported, including a changed expression of drug efflux pump P-gp.3 In addition, tubulin mutation may contribute to resistance to PTX therapy and inhibit activation of the apoptosis pathway.4,5 It is therefore essential to search for new breast cancer drug-resistance-related protein markers and improve the signal-transduction resistance regulation network to overcome this major disadvantage of PTX therapy. Abhijit Saha have designed a novel peptide–docetaxel conjugate, which delivers docetaxel specifically to cancer cell targeting neuropilin-1 receptor, thus enhancing its efficacy and acts more aggressively in breast cancer cells.6 Si has reported ERα propelled aberrant global DNA hypermethylation by activating the DNMT1 gene to enhance anticancer drug resistance in human breast cancer cells.7 Knockdown of MyD88 reduced the proliferation, migration, and invasion of MCF-7 cells and increased the sensitivity of MCF-7 cells to paclitaxel treatment through the inhibition of activation of NF-κB via PI3K/Akt.8Previous research in our laboratory, using the proteomic approach and two-dimensional gel electrophoresis to compare differential proteins between the human breast cancer paclitaxel-resistant cell line (MCF-7/PTX) and wild-type human breast cancer cells (MCF-7/S) revealed that SET (patient SE translation) is one of the most significantly altered proteins, with its expression being 5.8 times higher in MCF-7/PTX cells.9 We also found knockdown of SET by RNA interference led to the induction of apoptosis and suppression of cancer cell proliferation in those cells.10 These results suggested that SET could be a novel therapeutic molecular target for breast cancer. SET, which was first described in acute undifferentiated leukemia and is associated with several biological functions, belongs to a protein molecular nucleosome assembly partner family.11 SET is a potent inhibitor of the tumor suppressor protein phosphatase 2A (PP2A).12 Both PP2A and SET are generally expressed in multiple organs and participate in the regulation of histone acetylation, DNA replication and transcription, nucleosome assembly, and cell apoptosis.13 Numerous studies indicate that down-regulation of SET plays an important role in aberrant gene expression in human cancers. Boqun14 found that excessive SET expression in patients with polycystic ovarian syndrome was associated with a poor prognosis. Cervoni11 found that SET expression was more than twofold higher in cancerous tissue samples from the womb, stomach, colon, and rectum cancer tissues than in the corresponding normal tissues. Accumulated SET in Human Epithelial Kidney cells (HEK293T) could act via protein kinase B (Akt)/PTEN either as a cell survival signal or as an oxidative stress sensor for cell death.15 COG112, which is a protein inhibitor of SET, can inhibit tumor suppressor PP2A, and then reduce the downstream level of PP2A in the phosphatidylinositol 3-kinase (PI3K)/Akt and c-Myc signaling pathways, and also reduce the degree of apoptosis.16
Numerous studies indicate that down-regulation of acetylation and the deacetylation of histone play important roles in aberrant gene expression in human cancers. Histone deacetylases (HDACs) have thus become attractive therapeutic targets, and many HDACIs are actively under investigation as potential anticancer agents. Histone acetylation modifies specific lysine residues on histones and plays a key role in several cellular functions such as chromosome remodeling, gene transcription, and cell proliferation.17 Kim18 confirmed that a histone deacetylases inhibitor (HDACI) can regulate cell-cycle proteins and the expression of cell-cycle protein kinase inhibitors, inducing tumor cell G1 and G2 stagnation, and achieve the effect of inhibiting tumor cell growth and mitochondrial-mediated apoptosis. Down-regulation of histone acetylation can promote drug-resistant tumors. Recent studies have shown that suberoylanilide hydroxamic acid (SAHA) potentiates PTX-induced apoptosis and can overcome PTX resistance in ovarian cancer cell lines.19 The new anticancer drugs based on HDACIs using SAHA can induce endometrial gland cancer cell differentiation, which is characterized by change in morphology, glycogen synthesis, and the secretory phase of the expression of specific proteins.20 Chao21 confirmed that a low dose of LBH589 could increase the sensitivity of ovarian cancer cells to PTX by regulating the PI3K/Akt signaling pathway, releasing of cytochrome C and activating the mitochondrial apoptosis pathway. A previous study of LBH589 showed that this can promote the drug-resistant cell sensitivity to aromatase inhibitors (AI) and significantly reverse resistance to AI in MCF-7/S breast cancer cells by inhibiting the NF-κB signaling pathway.22 These studies have demonstrated that new HDACIs are potentially useful in antitumor field and for reversing drug resistance.
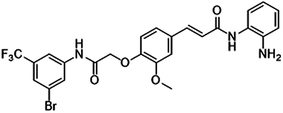
Both the overexpression of SET protein and HDAC exert critical effects on cell proliferation and apoptosis via different mechanisms, including dysregulation of the PI3K/Akt and apoptosis signaling pathways. Moreover, both can cause excessive expression of histone acetylation, which is subsequently develops into the source of chemotherapy resistance. We therefore hypothesized that applying FA18 (Fig. 1) a novel synthetic HDACI shown to possess a tumor suppressor function in a previous study23—in MCF-7/PTX cells may be able to overcome PTX resistance and enhance the efficacy of PTX against SET-overexpressing breast cancer cells by the down-regulation of SET expression and the inactivation of PI3K/Akt signaling.
Materials and methods
Materials and antibodies
Paclitaxel was purchased from Nanjing Sike Pharmaceutical Co., Ltd. (China). OP449 was from Michael P. Vitek as a gift (USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma (St Louis, USA). Dimethyl sulphoxide (DMSO) was from Tianjin Kemiou Chemical Reagent Co., Ltd. The annexin-V FLUOS staining kit was obtained from 4A Biotech (Beijing, China). RPMI-1640 and TRIzol reagent were purchased from Gibco Invitrogen (Carlsbad, CA), and fetal bovine serum was from Hangzhou Sijiqing Company (China). Rabbit antibodies against SET (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution), P-gp (1
2000 dilution), P-gp (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution) and PP2A (1
500 dilution) and PP2A (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 dilution) were from GeneTex (Irvine, CA, USA). Rabbit antibodies against multidrug resistance associated protein 1 (MRP1; 1
5000 dilution) were from GeneTex (Irvine, CA, USA). Rabbit antibodies against multidrug resistance associated protein 1 (MRP1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution) and breast cancer resistance protein (BCRP; 1
500 dilution) and breast cancer resistance protein (BCRP; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 dilution) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit antibodies against total Akt (t-Akt; 1
500 dilution) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Rabbit antibodies against total Akt (t-Akt; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), phosphorylated Akt (p-Akt; 1
1000 dilution), phosphorylated Akt (p-Akt; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution), B cell lymphoma (Bcl-2)-associated X protein (Bax; 1
1000 dilution), B cell lymphoma (Bcl-2)-associated X protein (Bax; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 dilution), Bcl-2 (1
2500 dilution), Bcl-2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2500 dilution), caspase 9 (1
2500 dilution), caspase 9 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution), caspase 3 (1
2000 dilution), caspase 3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution) and anti-poly adenosine diphosphate-ribose polymerase (PARP; 1
2000 dilution) and anti-poly adenosine diphosphate-ribose polymerase (PARP; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 dilution) were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit antibodies against β-actin (1
2000 dilution) were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit antibodies against β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 dilution) were obtained from Beijing Biosynthesis Biotechnology (Beijing, China). Horseradish peroxidase conjugated goat anti-rabbit secondary antibody (1
800 dilution) were obtained from Beijing Biosynthesis Biotechnology (Beijing, China). Horseradish peroxidase conjugated goat anti-rabbit secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dilution) was from CW biotech (Beijing, China).
000 dilution) was from CW biotech (Beijing, China).
Cell lines and cell culture
The human breast carcinoma cell line MCF-7 was obtained from the Cell Bank of Institute of Biochemistry and Cell Biology in Chinese Academy of Sciences (Shanghai, China). MCF-7/PTX cells were established in a stepwise escalating concentration manner from 2 to 30 nM paclitaxel with a continuous induction within 12 months.24 The reversal fold (RF) was 115. The MCF-7 cells and MCF-7/PTX cells were maintained in RPMI-1640 medium (Gibco) with 10% heat-inactivated fetal bovine serum (Gibco) and 1% penicillin/streptomycin, except that the culture conditions for the MCF-7/PTX cells were in the addition of 30 nM paclitaxel. Both the MCF-7 and MCF-7/PTX were cultured under a humidified atmosphere of 5% CO2 at 37 °C.MTT assay for cell viability
The growing cells were seeded at an initial density of 4 × 104 cells per well in 96-well plates along with overnight incubation at 37 °C and 5% CO2 with volumes of 100 μL medium. Subsequently, cells were treated with a series of concentrations of HDACI or prior to incubation in fresh culture medium containing paclitaxel for 72 h.Then, the medium were removed and 20 μL (5 mg mL−1) of MTT was added into each well and incubated for 4 h. Culture medium was also added to the control wells. The blue MTT formazan precipitate was then dissolved in 150 μL DMSO and the culture plates were gently agitated for 15 min. The absorbance values were detected in a plate reader at a wavelength of 492 nm in each well. The IC50 values were measured by GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The RF values were calculated as the IC50 of the cytotoxic drug/IC50 of the cytotoxic drug with test drug pretreatment.
RNA preparation and real-time quantitative polymerase chain reaction (qRT-PCR)
Cells from each experimental group were collected by centrifugation. Total mRNA was extracted with use of the RNAfast2000 kit (Fastagen, Shanghai, China) according to the manufacturer's protocol. Reverse transcription to cDNA was performed using the Superscript First Strand synthesis system (Bio-Rad, Richmond, USA). Real-time quantitative PCR was conducted with PrimeScript RT Master Mix Perfect Real Time kit (TaKaRa DRR036A) and SYBR Premix Ex Taq II (TaKaRa) following the manufacturer's instructions. The SET-forward primer sequence was GGAGGAAGATGAAGAGGCAT, and the SET-reverse primer sequence was TGGCTTTATTCTGCGTTTGAC. The conditions for qRT-PCR were performed as follows: 1 cycles of 95 °C for 30 s, 40 cycles of 95 °C for 5 s, various annealing temperature for 30 s depending on the target gene. [58 °C for SET and 56 °C for β-actin]. 60 °C for 30 s for cooling program. The mRNA expression in each sample was repressed as relative levels and was normalized to β-actin.Western blot analysis
Cells proteins were collected by centrifugation and washed twice with PBS and lysed in RIPA lysis buffer at 4 °C. Protein concentration was measured by BCA reagent (Beyotime, China). Then, the lysates were subjected to 10% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) at denaturing conditions and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA). Subsequently, the membranes were blocked with 5% nonfat dry milk in PBS for 2 h at room temperature and then incubated with primary antibodies overnight at 4 °C. The membranes were washed and the blots localization was labeled with horseradish peroxidase-conjugated secondary antibodies. The immunoreactive bands were detected using the ECL western blot analysis system (Amersham Biosciences). Experiments were independently performed in triplicate.Flow cytometry assay
Cells were harvested and washed and resuspended with cold phosphate buffered solution (PBS). Cells were double stained with V-FITC and propidium iodide (PI) in dark with an FITC/PI detection kit in accordance with the manufacture's instruction for 20 min at room temperature. The stained cells were detected by using FACSCanto™ II flow cytometry (Becton Dickinson Company, USA). Experiments were independently performed in triplicate.Immunohistochemistry
The xenograft tumors tissue sections were deparaffinized with xylene and dehydrated in declining grades of ethanol, then in pressure cooker antigen retrieval was performed at 121 °C for 5 min (10 mM citrate buffer, pH 6.0). Then, the endogenous peroxidases were blocked with 3% hydrogen peroxide for 10 min. The non-specific conjugation was blocked with 2% normal rabbit serum for 20 min and incubated with primary antibody of SET (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 dilution) overnight at 4 °C. Then, the tissue sections were incubated with the species-specific secondary antibody and followed by 3,3′-diaminobenzidine developing, and then counterstained with Harris' modified hematoxylin. The immunostaining intensities or percentages of positive cells were measured under bright-field microscopy follow previously described.
200 dilution) overnight at 4 °C. Then, the tissue sections were incubated with the species-specific secondary antibody and followed by 3,3′-diaminobenzidine developing, and then counterstained with Harris' modified hematoxylin. The immunostaining intensities or percentages of positive cells were measured under bright-field microscopy follow previously described.
Tumor xenografts in nude mice
Twenty Male BALB/c nude mice at four weeks of age were purchased from Shanghai Laboratory Animal center of the Chinese Academy of Sciences and maintained in a pathogen free animal facility in accordance with the Institutional Animal Care and Use Committee of Xi'an Jiaotong University. All mice were injected subcutaneously with MCF-7 in at a density of 1 × 107 cells in 200 μL in the subdermal space on the right to induce breast tumor xenografts. Tumor size was measured on alternate days by using a vernier caliper and calculated by length × width2 × 0.5 (in cm3). Once tumors grew to no less than 0.1 cm3 in volume, mice were randomly divided into four groups and treated intraperitoneally with vehicle, PTX (9 mg kg−1), HDACI (10 mg kg−1) or in combination every 3 days for a total of seven times. After a total of 21 days of treatment, mice were sacrificed and tumor tissue was harvested, weighed. One part of the tissue were preserved in liquid nitrogen and extracted for western blot. The other parts were fixed in 4% paraformaldehyde for immunohistochemical analysis.Statistical analyses
Results represented are expressed as the means ± standard deviation (SD). Statistical analysis of the data was performed using SPSS 19.0 (SPSS, Chicago, IL, USA). Statistical comparisons were performed with one-way ANOVA. A value of p < 0.05 was considered to indicate a statistically significant difference.Results
Evaluation of the SET/PP2A/Akt signaling pathway in MCF-7/PTX cells
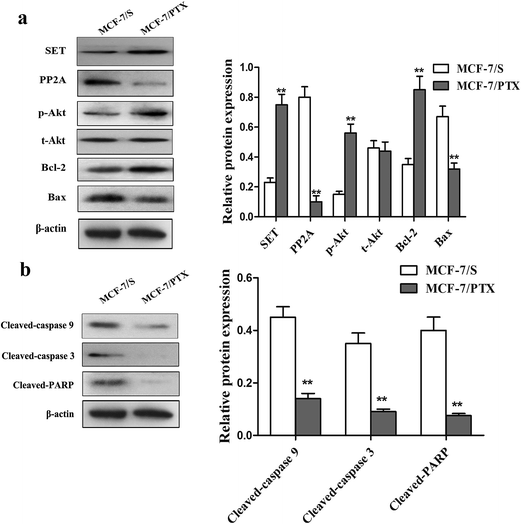
Based on previous studies, up-regulation of SET is thought to account for the chemoresistance phenotype.10 SET protein is an inhibitor of PP2A, which regulates the levels of p-Akt and is required for cell growth. Greater phosphorylation of Akt improves cell proliferation and survival by regulating downstream molecules that mediate the cell cycle and apoptosis. In addition, activation of the PI3K/Akt signaling pathway plays a critical role in mediating the mitochondria-regulated intrinsic cell-death pathway, and this has been described in various cancers and may induce resistance to chemotherapy treatment. In order to clarify whether SET, PP2A, and the PI3K/Akt signaling pathway are associated with drug resistance in MCF-7/PTX cells, we tested for differences in their expression levels between MCF-7/S and MCF-7/PTX cells.Fig. 2 shows that SET, p-Akt, and Bcl-2 proteins were overexpressed, while the expression levels of PP2A and Bax proteins were significantly reduced in MCF-7/PTX cells compared with MCF-7/S cells. Caspase-9, caspase-3, and PARP are key factors of mitochondrial apoptosis pathways, which are regulated by the PI3K/Akt signaling pathway.2 Therefore, the activation levels of caspase-9, caspase-3, and PARP were investigated with the aim of identifying mechanisms underlying PTX-mediated resistance in breast cancer cells. The results indicated that the activated SET/PP2A/Akt signaling pathway and mitochondrial apoptosis pathways may be involved in the formation of chemoresistance in breast cancer.
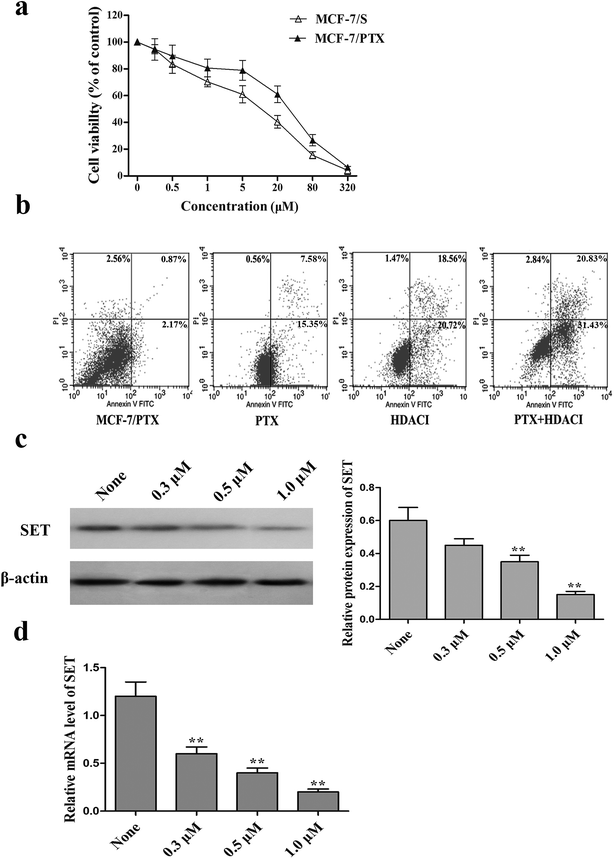
Down-regulation of SET protein in MCF-7/PTX cells increases PTX-induced apoptosis
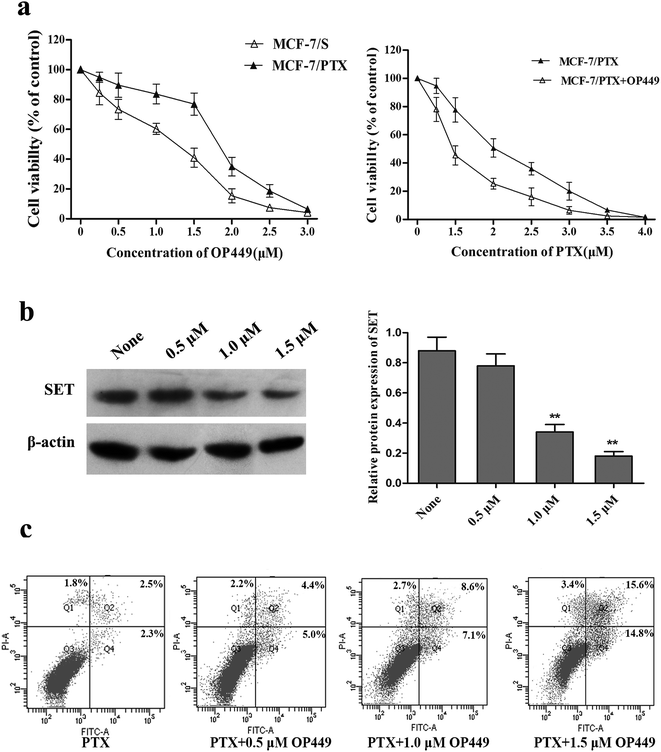
Elevated SET protein levels may account for PTX resistance in breast cancer MCF-7/PTX cells, and reducing the expression of SET might enhance the sensitivity of these cells to PTX. OP449 is a specific inhibitor of SET; this is a kind of peptide that binds directly to SET and antagonizes its function.25 Our Growth-inhibition experiment showed that OP449 can reduce the survival rates of MCF-7/S and MCF-7/PTX cells in a concentration-dependent manner. To evaluate the effect of OP449 on the sensitivity to PTX, MCF-7/PTX cells were exposed to PTX in the presence of 1.5 μM OP449 (inhibition rate < 20%). The experimental results showed that OP449 remarkably enhances the cytotoxicity of MCF-7/PTX cells to PTX. Fig. 3 shows that pretreatment of MCF-7/PTX cells with OP449 reduced the protein levels of SET. Based on the western blot results, we found this finding can justified OP449 could interrupt SET at the level of translation and down-regulation of SET protein expression to antagonize its function.In a further evaluation of the involvement of SET in PTX-induced apoptosis in MCF-7/PTX cells, flow-cytometry results indicated that the number of apoptotic cells in the group treated with 600 nM PTX increased sharply after pretreatment with 0.5 μM, 1.0 μM, 1.5 μM OP449 compared to that applying PTX alone in dose-dependent manner. These results confirm that using an inhibitor to decrease SET can enhance the sensitivity of MCF-7/PTX cells to PTX.
SET mediates PTX-induced apoptosis in MCF-7/PTX cells via the PI3K/Akt signaling pathway
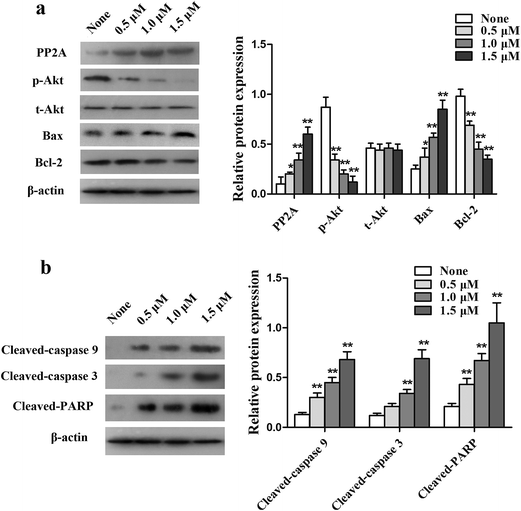
To further explore the potential mechanisms underlying SET-induced PTX resistance, western blot analysis was applied to detect the level of PI3K/Akt and the mitochondrial apoptosis pathways in MCF-7/PTX cells. Fig. 4 shows that treatment with OP449 resulted in MCF-7/PTX cells expressing more PP2A protein compared with the not-treated group. Western blot analysis showed that the level of p-Akt was significantly reduced in MCF-7/PTX cells pretreated with OP449, but did not apparently influence the expression of t-Akt. A similar trend was detected in the protein expression of Bcl-2, whereas the level of Bax was significantly increased. Meanwhile, cleaved caspase-9, cleaved caspase-3, and cleaved PARP were elevated compared with the parental cells. These results indicate that SET induces drug resistance in MCF-7/PTX cells by activating the PI3K/Akt signaling pathway.HDACI potentiates the sensitivity of MCF-7/PTX cells to PTX
The intrinsic cytotoxicity of the HDACI in MCF-7 and MCF-7/PTX cells was evaluated using the MTT assay. MCF-7 and MCF-7/PTX cells were treated with the HDACI for 48 h. Fig. 5a shows that the HDACI inhibited the growth of MCF-7/S and MCF-7/PTX cells in a dose-dependent manner. The data suggest that MCF-7/PTX cells do not exhibit resistance to the HDACI. To investigate the reversal efficacy of the HDACI whilst exerting minimal effects on cell vitality, it was applied at concentrations of 0.3, 0.5, and 1.0 μM (the last corresponds to the concentration for which the inhibition was <15%) in order to produce cell viability curves. MTT assays and reversal index (RI) were determined to examine whether the HDACI reversed the resistance of MCF-7/PTX cells to PTX.As indicated in Table 1, after 48 h of treatment with the HDACI at 0.3, 0.5, and 1.0 μM, the RI values were 2.3, 3.5, and 4.8, respectively. These RI values indicated the potency of reversal, with values >1 indicating that the drug sensitized the MCF-7/PTX cells to PTX. Meanwhile, a flow-cytometry experiment was carried out to ascertain the effects of the HDACI on the PTX-induced cytotoxic effects in MCF-7/PTX cells treated with PTX and the HDACI. Compared with the group in which the drugs were applied separately, combining PTX and the HDACI clearly increased the number of apoptotic MCF-7/PTX cells. It therefore appears that the new HDACI has the ability to enhance the PTX-induced apoptosis in MCF-7/PTX cells.
| Group | Concentration (μM) | IC50 of PTX (nM) | RF |
|---|---|---|---|
| a The effect of FA18 on the sensitivity of MCF-7/PTX cells to PTX was determined by MTT method. MCF-7/PTX cells were treated with various concentrations of paclitaxel in the presence of FA18 at the indicated concentration for 48 h. IC50 for paclitaxel and RI values were calculated. Results are presented as mean ± SD from three independent experiments. | |||
| Control | 2290.87 ± 125.2 | ||
| FA18 | 0.3 | 964.3 ± 86.5 | 2.3 |
| 0.5 | 633.5 ± 70.2 | 3.5 | |
| 1.0 | 462.7 ± 58.6 | 4.8 | |
Since modulation of the SET/PI3K/Akt signaling pathway plays an important role in mediating the PTX resistance in MCF-7/PTX cells, we also assessed the effect of the HDACI on SET using qRT-PCR and western blot analysis. Fig. 5c and d shows that treatment with the HDACI significantly reduced the gene and protein expressions of SET in a dose-dependent manner. This indicates that the HDACI inhibits the expression of SET at the transcription level in MCF-7/PTX cells, and potentiates the sensitivity of MCF-7/PTX cells to PTX via the suppression of SET.
HDACI reverses PTX resistance by inhibiting the SET/PI3K/Akt signaling pathway
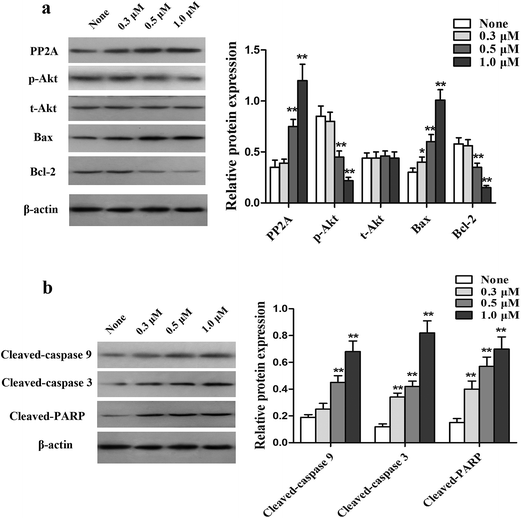
Activation of the PI3K/Akt signaling pathway is a common cause of apoptosis resistance. To examine whether this pathway is involved in mediating the PTX resistance in MCF-7/PTX cells, PP2A, p-Akt, Bcl-2, and Bax in MCF-7/PTX cells were first determined by western blot analysis. Fig. 6a shows that treatment with the HDACI for 48 h congruously decreased the protein level of p-Akt along with Bcl-2, and with a concomitant augmentation of the expressions of PP2A and Bax, but did not apparently influence the expression of t-Akt. The expression levels of downstream mitochondrial apoptosis pathway molecules—cleaved caspase-9, cleaved caspase-3, and cleaved PARP were detected to further assess the inhibitory action of the HDACI on cell apoptosis. Fig. 6b shows that the expression levels of cleaved caspase-9, cleaved caspase-3, and cleaved PARP were increased after HDACI treatment. These results indicate that the HDACI overcomes PTX resistance by inhibiting the SET/PI3K/Akt signaling pathway.HDACI sensitizes MCF-7/PTX-cell xenografts to PTX treatment
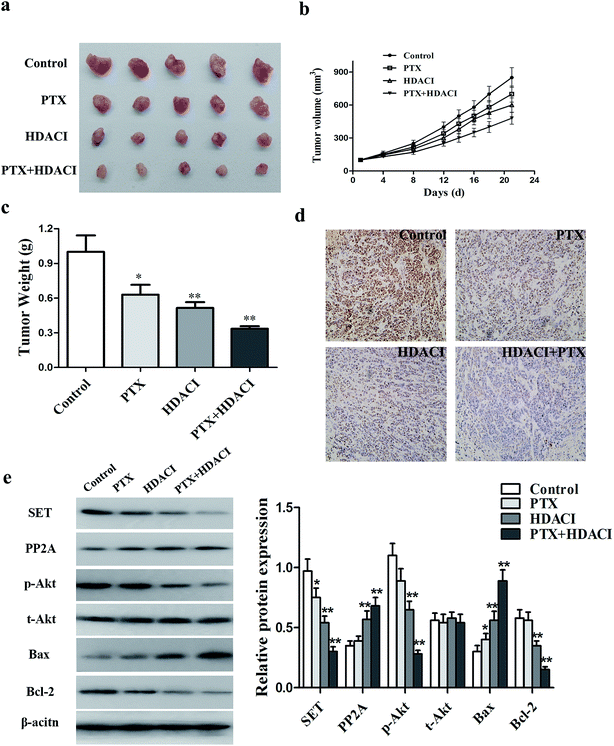
To elucidate whether the HDACI could reverse PTX resistance in vivo, we examined the effects of the HDACI in a breast tumor animal model by subcutaneously implanting MCF-7/PTX cells into mice. When the tumor size reached 100 mm3, mice (five per group) were treated with vehicle, PTX (10 mg kg−1), FA18 (9 mg kg−1), or both PTX and FA18 intraperitoneally every 3 days a total of seven times. The tumors from mice that received the combination treatment were clearly smaller and less heavy than those from either the HDACI or PTX treatment group. The combination of the HDACI and PTX significantly potentiated PTX-mediated tumor growth inhibition by reducing the tumor size by 48.0%. In addition, the drug therapy resulted in no conspicuous reductions in body weight and any other abnormities. Immunohistochemical analysis was also applied to detect how the HDACI and PTX treatment influenced the SET/PP2A/Akt signaling pathway in tumor tissues of nude mice. The expression of SET was almost undetectable in the combinational treatment, in contrast to strong positive expression in the control group. At the molecular level, the HDACI combined with PTX treatment significantly inhibited SET expression and the PI3K/Akt signaling pathway in the tumor tissues. These results indicate that the HDACI can down-regulate the SET/PI3K/Akt signaling pathway to reverse the PTX resistance of breast cancer cells in vivo (Fig. 7).Discussion
PTX, which stabilizes microtubules to reduce their dynamicity and promote mitotic arrest and eventually apoptosis, was first isolated from the bark of pacific yew and is widely used in cancer chemotherapies.26 However, the development of PTX resistance is frequently observed in patients and poses a major obstacle to successful therapy. Therefore, identifying the molecular mechanisms underlying PTX resistance and developing novel agents with definite anti-resistance activity are of considerable interest in breast cancer. The differential expression of P-gp, tubulin mutation and inhibition of the apoptosis pathway has been reported as causes of clinical drug resistance to PTX.SET was first discovered as a component of the set-can fusion gene produced by a somatic translocation in a case of acute undifferentiated leukemia.27 SET positively regulates multiple oncogenic pathways and is involved in histone acetylation, cell apoptosis, transcriptional regulation, nucleosome assembly, and various normal biological processes. SET overexpression in HEK293 cells promotes mitochondrial fission and reduces the autophagic flux in an apparent association with up-regulation of UCP2 and UCP3 to induce cancer.28 SET also inhibits the expression of its downstream tumor suppressor factor, PP2A, and activates the PI3K/Akt signaling pathway in head and neck squamous cell carcinoma.29 The active form of Akt improves cell proliferation by regulating downstream effectors that mediate cell apoptosis. Strong activity of the PI3K/Akt signaling pathway can inhibit apoptosis, down-regulate the pro-apoptosis molecule Bax and upregulate the downstream pro-survival molecule Bcl-2 (which is now the most-studied of the cell-apoptosis genes). Bcl-2 and Bax are frequently targeted genes that regulate cell apoptosis.
In this study we tested the potential therapeutic role for a SET antagonist in breast cancer, which is the most common type of tumor in women. We examined the activation of the SET/PI3K/Akt signaling pathway in breast cancer cell lines, and observed a higher level of expression in MCF-7/PTX cells than in MCF-7/S cells. The SET-specific antagonist OP449 induced apoptosis in MCF-7/PTX cells and down-regulated the SET protein level, as well as up-regulated PP2A. PP2A is downstream of the PI3K/Akt signaling pathway, and it has been shown to play an important role in several tumor-promoting pathways, and is frequently down-regulated in human cancers. PP2A directly dephosphorylates Akt, resulting in the inhibition of the pro-survival pathways and overall growth retardation. Therefore, OP449 treatment remarkably inhibited the Akt signaling pathway, and the cleaved caspase-9, cleaved caspased-3, and cleaved PARP were subsequently activated in a concentration-dependent manner.
The use of reversal agents has been an active area of research into antitumor and drug resistance for several years. As a structural protein of histone, SET possesses important biological functions that could enhance deacetylation and affect histone posttranslational modification, and these characteristics could be inhibited by an HDACI.30 Studies have shown that the new HDACI could activate caspase-3, inhibit survivin protein, and induce cell apoptosis; moreover, it could block Akt and the MAPK signal transduction pathway, regulate Bax and Bcl-2, and inhibit the proliferation and migration of tumor cells.31,32 However, no previous studies have elucidated whether SET participates in this or if an HDACI could be used in the reversal of drug resistance in breast cancer.
In the present study, we found that the new HDACI FA18 can reduce the survival rates of MCF-7/S and MCF-7/PTX cells in a concentration-dependent manner, and that low doses of FA18 can promote PTX-induced apoptosis in MCF-7/PTX cells and increase the sensitivity of MCF-7/PTX cells to PTX. More importantly, we found the new HDACI can reduce the gene and protein levels of SET, indicating the involvement of SET in the PTX resistance. To explore its reversal mechanism, we found that the HDACI can promote PP2A protein expression and Bax, and reduce the phosphorylation of Akt and Bcl-2 in MCF-7/PTX cells. Additionally, the HDACI mediated mitochondrial apoptosis pathways and promoted the activation of members of the caspase family, then accelerated the decomposition of PARP. The results showed that the HDACI induced cell apoptosis and eventually reversed PTX resistance in breast cancer by inhibiting the SET/PP2A/Akt signaling pathway.
In order to verify the results of in vivo experiments, this study also established the MCF-7/PTX cell transplantation tumor in nude mouse model. It was found that compared with treatment with either HDACI or PTX alone, the tumor growth in nude mice when using a combination of HDACI and PTX was slower and the resulting tumor was smaller; furthermore, down-regulation of the SET level and activity of the PI3K/Akt signaling pathway were both observed in xenografted tumors containing MCF-7/PTX cells in the combination group. These findings suggest that the new HDACI has a potent ability to reverse PTX resistance in breast cancer, and hence it represents an effective candidate compound for overcoming breast cancer resistance. In contrast to other studies of HDACIs in cancer research, we used an HDACI as the reversal agent to overcome PTX resistance in breast cancer and have discussed the mechanisms of how an HDACI can reverse PTX resistance. However, there are numerous key downstream targets of the PI3K/Akt signaling pathway, including mammalian targets of rapamycin and p70S6 kinase, which participate in regulating apoptosis.33 Therefore, further studies are required to fully elucidate the reversal mechanisms of HDACIs. Interestingly, there is also an inhibitor of SET (FTY720) that can inhibit SET in cancer cells;34 whether it could present an effective inhibitor in MCF-7/PTX cells remains to be determined.
In summary, our findings have revealed a previously unrecognized role of SET in PTX resistance, and have shown that a new HDACI can significantly enhance the inhibition of cell growth induced by PTX and cell apoptosis in SET-overexpressed MCF-7/PTX cells. Major anticancer activities of FA18 include down-regulation of SET protein levels and inactivation of the PI3K/Akt signaling pathway. The HDACI FA18 may represent a novel therapeutic tool for preventing or reversing resistance to PTX in breast cancer.
Conflict of interest
We declare that we have no conflict of interest.Acknowledgements
The work is supported by National Natural Science Foundation of China (No. 81473177).References
- N. Bhoo-Pathy, C. H. Yip, M. Hartman, C. S. Uiterwaal, B. C. Devi, P. H. Peeters, N. A. Taib, C. H. van Gils and H. M. Verkooijen, Eur. J. Cancer, 2013, 49, 703–709 CrossRef PubMed.
- J. Cai, S. Chen, W. Zhang, X. Zheng, S. Hu, C. Pang, J. Lu, J. Xing and Y. Dong, Phytomedicine, 2014, 21, 1725–1732 CrossRef CAS PubMed.
- X. Yang, J. Shen, Y. Gao, Y. Feng, Y. Guan, Z. Zhang, H. Mankin, F. J. Hornicek and Z. Duan, Int. J. Cancer, 2015, 137, 2029–2039 CrossRef CAS PubMed.
- S. Yin, C. Zeng, M. Hari and F. Cabral, Cytoskeleton, 2013, 70, 849–862 CrossRef CAS PubMed.
- S. Sharifi, J. Barar, M. S. Hejazi and N. Samadi, Asian Pacific Journal of Cancer Prevention, 2014, 15, 8617–8622 CrossRef PubMed.
- A. Saha, S. Mohapatra, P. Kurkute, B. Jana, J. Sarkar, P. Mondal and S. Ghosh, RSC Adv., 2015, 5, 92596–92601 RSC.
- X. Si, Y. Liu, J. Lv, H. Ding, X. A. Zhang, L. Shao, N. Yang, H. Cheng, L. Sun, D. Zhu, Y. Yang, A. Li, X. Han and Y. Sun, Oncotarget, 2016 DOI:10.18632/oncotarget.8038.
- F. Xiang, Z. Ni, Y. Zhan, Q. Kong, J. Xu, J. Jiang, R. Wu and X. Kang, Tumour Biol., 2015 DOI:10.1007/s13277-015-4436-5.
- S. Chen, Q. Dong, S. Hu, J. Cai, W. Zhang, J. Sun, T. Wang, J. Xie, H. He, J. Xing, J. Lu and Y. Dong, Mol. BioSyst., 2014, 10, 294–303 RSC.
- W. Zhang, J. Cai, S. Chen, X. Zheng, S. Hu, W. Dong, J. Lu, J. Xing and Y. Dong, Mol. Med. Rep., 2015, 12, 1506–1514 CAS.
- N. Cervoni, N. Detich, S. B. Seo, D. Chakravarti and M. Szyf, J. Biol. Chem., 2002, 277, 25026–25031 CrossRef CAS PubMed.
- I. Cristobal, P. Gonzalez-Alonso, L. Daoud, E. Solano, B. Torrejon, R. Manso, J. Madoz-Gurpide, F. Rojo and J. Garcia-Foncillas, Mar. Drugs, 2015, 13, 3276–3286 CrossRef CAS PubMed.
- H. Liu, Y. Gu, H. Wang, J. Yin, G. Zheng, Z. Zhang, M. Lu, C. Wang and Z. He, Oncotarget, 2015, 6, 14913–14925 CrossRef PubMed.
- X. Boqun, D. Xiaonan, C. Yugui, G. Lingling, D. Xue, C. Gao, D. Feiyang, L. Jiayin, L. Gao, M. Li, Y. Zhang and X. Ma, Int. J. Endocrinol., 2013, 2013, 367956 Search PubMed.
- A. M. Leopoldino, C. H. Squarize, C. B. Garcia, L. O. Almeida, C. R. Pestana, A. C. Polizello, S. A. Uyemura, E. H. Tajara, J. S. Gutkind and C. Curti, Mol. Cell. Biochem., 2012, 363, 65–74 CrossRef CAS PubMed.
- C. H. Switzer, R. Y. Cheng, T. M. Vitek, D. J. Christensen, D. A. Wink and M. P. Vitek, Oncogene, 2011, 30, 2504–2513 CrossRef CAS PubMed.
- V. Carafa, M. Miceli, L. Altucci and A. Nebbioso, Expert Opin. Ther. Pat., 2013, 23, 1–17 CrossRef CAS PubMed.
- S. O. Kim, B. T. Choi, I. W. Choi, J. Cheong, G. Y. Kim, T. K. Kwon, N. D. Kim and Y. H. Choi, BMB Rep., 2009, 42, 655–660 CrossRef CAS PubMed.
- A. Angelucci, M. Mari, D. Millimaggi, I. Giusti, G. Carta, M. Bologna and V. Dolo, Gynecol. Oncol., 2010, 119, 557–563 CrossRef CAS PubMed.
- H. Uchida, T. Maruyama, M. Ono, K. Ohta, T. Kajitani, H. Masuda, T. Nagashima, T. Arase, H. Asada and Y. Yoshimura, Endocrinology, 2007, 148, 896–902 CrossRef CAS PubMed.
- H. Chao, L. Wang, J. Hao, J. Ni, L. Chang, P. H. Graham, J. H. Kearsley and Y. Li, Cancer Lett., 2013, 329, 17–26 CrossRef CAS PubMed.
- M. Kubo, N. Kanaya, K. Petrossian, J. Ye, C. Warden, Z. Liu, R. Nishimura, T. Osako, M. Okido, K. Shimada, M. Takahashi, P. Chu, Y. C. Yuan and S. Chen, Breast Cancer Res. Treat., 2013, 137, 93–107 CrossRef CAS PubMed.
- F. Wang, W. Lu, T. Zhang, J. Dong, H. Gao, P. Li, S. Wang and J. Zhang, Bioorg. Med. Chem., 2013, 21, 6973–6980 CrossRef CAS PubMed.
- S. Y. Chen, S. S. Hu, Q. Dong, J. X. Cai, W. P. Zhang, J. Y. Sun, T. T. Wang, J. Xie, H. R. He, J. F. Xing, J. Lu and Y. L. Dong, Asian Pacific Journal of Cancer Prevention, 2013, 14, 6135–6140 CrossRef PubMed.
- A. Agarwal, R. J. MacKenzie, R. Pippa, C. A. Eide, J. Oddo, J. W. Tyner, R. Sears, M. P. Vitek, M. D. Odero, D. J. Christensen and B. J. Druker, Clin. Cancer Res., 2014, 20, 2092–2103 CrossRef CAS PubMed.
- D. Zhang, R. Yang, S. Wang and Z. Dong, Drug Des., Dev. Ther., 2014, 8, 279–284 CAS.
- Y. Adachi, G. N. Pavlakis and T. D. Copeland, J. Biol. Chem., 1994, 269, 2258–2262 CAS.
- L. O. Almeida, R. N. Goto, M. P. Neto, L. O. Sousa, C. Curti and A. M. Leopoldino, Biochem. Biophys. Res. Commun., 2015, 458, 300–306 CrossRef CAS PubMed.
- T. Mazumdar, L. A. Byers, P. K. Ng, G. B. Mills, S. Peng, L. Diao, Y. H. Fan, K. Stemke-Hale, J. V. Heymach, J. N. Myers, B. S. Glisson and F. M. Johnson, Mol. Cancer Ther., 2014, 13, 2738–2750 CrossRef CAS PubMed.
- S. B. Seo, P. McNamara, S. Heo, A. Turner, W. S. Lane and D. Chakravarti, Cell, 2001, 104, 119–130 CrossRef CAS PubMed.
- J. J. Hwang, Y. S. Kim, M. J. Kim, S. Jang, J. H. Lee, J. Choi, S. Ro, Y. L. Hyun, J. S. Lee and C. S. Kim, Anti-Cancer Drugs, 2009, 20, 815–821 CrossRef CAS PubMed.
- L. Bastian, J. Hof, M. Pfau, I. Fichtner, C. Eckert, G. Henze, J. Prada, A. von Stackelberg, K. Seeger and S. Shalapour, Clin. Cancer Res., 2013, 19, 1445–1457 CrossRef CAS PubMed.
- S. Ponnurangam, D. Standing, P. Rangarajan and D. Subramaniam, Mol. Cancer Ther., 2013, 12, 598–609 CrossRef CAS PubMed.
- D. Kubota, A. Yoshida, A. Kawai and T. Kondo, J. Proteome Res., 2014, 13, 2250–2261 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |