Construction of highly sensitive surface-enhanced Raman scattering (SERS) nanosensor aimed for the testing of glucose in urine†
Abstract
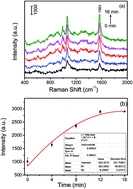
A facile, highly sensitive “turn on” surface-enhanced Raman scattering (SERS) nanosensor for the detection of glucose has been developed based on the aggregation of 4-mercaptophenylboronic acid (4-MPBA)-decorated silver nanoparticles in specific bonding to glucose, which can be used for the clinical detection of glucose in urine.

 Please wait while we load your content...
Please wait while we load your content...