Stability and bonding of the multiply coordinated bimetallic boron cycles: B8M22−, B7NM2 and B6C2M2 with M = Sc and Ti†
Abstract
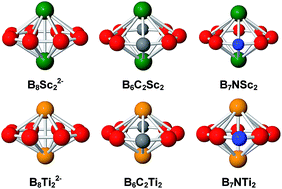
A theoretical investigation of the geometry, stability and aromaticity of boron clusters doped by two Sc and Ti atoms was carried out using DFT calculations. The Sc and Ti atoms form the bimetallic boron cycles not only with the eight-membered ring B82− but also with the isoelectronic species B7N and B6C2 rings yielding the planar B82−, B7N and B6C2 rings sandwiched to two transition metals. The N and C atoms prefer to form eight-membered hetero-rings via the classical 2e–2c bonding rather than occupy high coordination positions. Both C atoms avoid binding each other. The thermodynamic stability of all bimetallic boron cycles is induced by the stabilizing overlap between the bonding and anti-bonding MOs of a metallic dimer M2 with levels of an eight-membered ring. These stabilizing interactions also release two sets of delocalized σ and π MOs, which obey the (4N + 2) electron count. Such a double σ and π aromaticity feature is clearly supported by the magnetic ring current flows.

 Please wait while we load your content...
Please wait while we load your content...