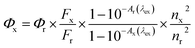Synthesis, structure and photophysical properties of near-infrared 3,5-diarylbenzoBODIPY fluorophores†
Jun Wang,
Qinghua Wu,
Yajun Xu,
Changjiang Yu,
Yun Wei,
Xiaolong Mu,
Erhong Hao* and
Lijuan Jiao*
The Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Laboratory of Molecule-Based Materials, School of Chemistry and Materials Science, Anhui Normal University, Wuhu, 241000, China. E-mail: haoehong@ahnu.edu.cn; jiao421@ahnu.edu.cn
First published on 23rd May 2016
Abstract
A set of 3,5-diarylated benzoBODIPYs were prepared within three steps from commercial isoindolin-1-one via Vilsmeier Haack, Suzuki coupling and a one-pot POCl3-promoted self-condensation in concert with a BF3 complexation. These benzoBODIPYs showed strong absorption and intense fluorescence emission in far-red to NIR region (λmaxabs = 634–706 nm, λmaxem = 663–747 nm, Φ = 0.35–0.99) tunable via the variation of the 3,5-aryl substituents.
Introduction
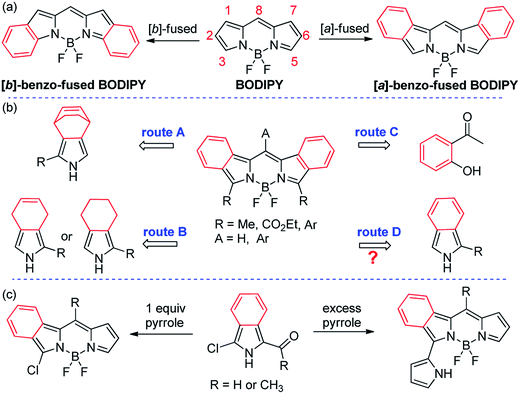
Near-infrared (NIR) organic chromophores,1 especially those with high molar absorptivities, bright fluorescence and good stabilities have attracted wide research interest over the past decades and have found applications in materials and biomedicine areas, for example as efficient photosensitizers in organic photovoltaics2 and as labeling/imaging agents in biological related systems.3 Boron dipyrromethenes (BODIPYs, Fig. 1) have excellent photophysical properties, like bright fluorescence and excellent photostability,4 and have been applied in a highly diverse research fields, including photovoltaics,5 bioimaging,6 sensing,7 and photodynamic therapy.8 Conventional BODIPYs generally absorb/emit between 470 and 530 nm.4 Long-wavelength NIR BODIPYs can be achieved via structural modifications,9,10 for example, through extending the conjugation at 3,5-positions,10–13 and the annulations of aromatic compounds onto the pyrrolic positions of the chromophore,14–19 for example the annulations of benzene-moieties to form the so-called “benzoBODIPYs”.20–24 Theoretically, there are two isomeric structures of benzoBODIPYs ([a] and [b]-fusion, Fig. 1a) with the annulations occurred at a and b-bond positions. Most benzoBODIPYs appeared in the literature are [a]-benzoBODIPYs21–24 with intense fluorescence emission that is in great contrast to the dark fluorescence observed in [b]-benzene-annulated isomers.20Their synthesis were generally achieved via: (i) a thermal retro Diels–Alder reaction (above 200 °C, route A, Fig. 1b) developed by Ono and coworker,21 in which the preparation of bicyclo-octadiene-fused pyrrole precursor was required, (ii) the condensation of di- and tetrahydroisoindoles reported by Vicente and coworkers,22 in which the oxidative dehydrogenation with DDQ was required (route B, Fig. 1b), and (iii) the total synthesis from 2-acetylphenols23 (route C, Fig. 1b). On the other hand, the direct condensation of isoindoles (route D, Fig. 1b) although straightforward is still inaccessible due to the stability issue of the dipyrromethene intermediate.
Recently, we have applied 3-chloro-1-formyl(acetyl)isoindoles for the condensation with pyrrole in the synthesis of unsymmetrical [a]-benzoBODIPYs and pyrrolylbenzoBODIPYs (Fig. 1c).24 Herein we report a three-step synthesis and photophysical properties of brightly NIR fluorescent 3,5-arylated benzoBODIPYs from commercial reagents based on an POCl3-promoted self-condensation of 3-aryl-1-formylisoindoles (Scheme 1).
 | ||
| Scheme 1 Synthesis of 3,5-diarylbenzoBODIPYs 1a–e from commercial isoindolin-1-one via Vilsmeier–Haack, Suzuki coupling and POCl3-promoted self-condensation reactions. | ||
Results and discussion
Synthesis
Our initial attempts to symmetrical 3,5-dihalogenobenzoBODIPY 1X from an acid promoted self-condensation25 of 3-halogeno-1-formylisoindole in POCl3/dichloromethane or HCl/methanol (Scheme S1†) failed. Instead, only a complicated polar mixture was obtained. We attributed it to the poor stability of the 3,5-dihalogenobenzodipyrromethene intermediate. We rationalized that the converting of 3-halogeno substituent, like bromo to aryl group may be helpful for the stabilization of the dipyrromethene intermediate and may provide an opportunity to get the direct access to symmetrical [a]-benzoBODIPYs.The key synthetic precursor 3 24,26 was generated in 92% yield from commercial isoindolin-1-one via Vilsmeier–Haack reaction (Scheme 1). The Suzuki coupling on 3 with 4-methoxyphenylboronic acid smoothly generated the desired 3-(4-methoxyphenyl)-1-formylisoindole 2a as a stable light yellow in 72% yield. Subsequent POCl3-promoted self-condensation of 2a in dichloromethane smoothly proceeded at room temperature and generated the target 3,5-di(4-methoxyphenyl) benzoBODIPYs 1a in 64% isolated yield. Therefore, the installation of aryl group at 3-position of 1-formylisoindole framework indeed stabilized the thus formed dipyrromethene intermediate, which can even be isolated from the reaction mixture.
To test the versatility of this reaction, we further prepared 3-aryl-1-formylisoindole 2b–d in 53–83% isolated yields from the Suzuki coupling of 3 with substituted phenylboronic acid and 5-thiophene-2-boronic acid. Subsequent self-condensation of 2b–d smoothly proceeded. After subsequent BF3 complexation with N,N-diisopropylamine (DIPA) and BF3·Et2O, the desired 3,5-diarylbenzoBODIPYs 1b–d were generated in 22–53% overall yields started from commercial isoindolin-1-one. Compounds 2a–e and 3,5-diarylbenzoBODIPYs 1a–e were characterized via NMR and HRMS. BenzoBODIPYs 1a, 1c and 1d were further characterized by X-ray analysis.
X-ray structure
Crystals of benzoBODIPYs 1a, 1c and 1d suitable for X-ray analysis were obtained by slow diffusion of methanol into their dichloromethane solutions at room temperature. As shown in Fig. 2, all these benzoBODIPYs showed nearly planar dipyrrin structures with dihedral angles between the two pyrrole units less than 6.4° (Table S2, ESI†). This nearly planar benzoBODIPY core results in the well extended π-conjugation and the observed bathochromic shift of the spectra. The dihedral angles between 3,5-phenyls and the dipyrrin core is in the range of 40.8° to 56.9° (Table S2, ESI†), indicates the intramolecular hydrogen bondings between F atoms and the hydrogen atoms on 3,5-phenyl moieties. Multiple intermolecular C–H⋯F hydrogen bonds between F atoms and various hydrogen atoms are formed due to the strong electron negativity of the F atom. These intermolecular hydrogen bondings help the establishment of the crystal packing structure and make these BODIPYs nearly parallel to each other in a head-to-tail orientation (Fig. S2, S4, and S6, ESI†).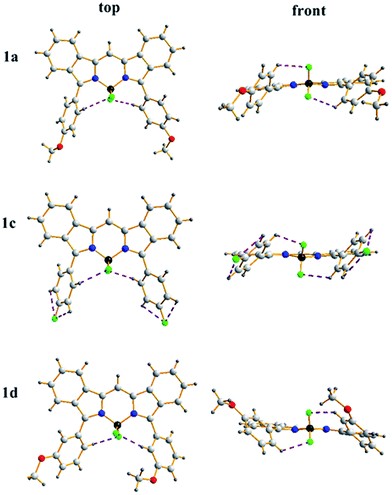 | ||
| Fig. 2 Top and front views of the X-ray structures of 1a, 1c and 1d. C, light gray; H, gray; N, blue; B, dark yellow; F, bright green; O, red. | ||
Photophysical properties
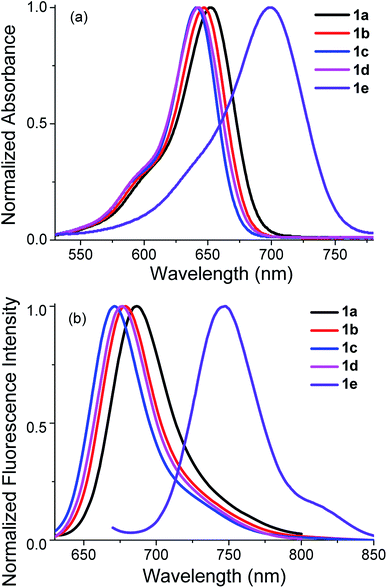
These 3,5-diarylated benzoBODIPYs are soluble in common organic solvents, like toluene, dichloromethane, THF, methanol and acetonitrile. Their absorption and emission spectra in these solvents were studied as summarized in the Table 1 and S3 (ESI).† As shown in Fig. 3, benzoBODIPYs 1a–e each showed a characteristic absorption and emission band similar to most BODIPYs,4 with the maximal absorption ascribed to the S0 → S1 (π–π*) transition. In dichloromethane, benzoBODIPYs 1a–e each showed strong absorption between 634 and 706 nm, and bright NIR fluorescence emission in the range of 663 and 747 nm. In comparison with their non-fused BODIPY analogs,27,28 these benzoBODIPYs each showed more than 80 nm bathochromic shift in both their absorption and emission spectra. The longest absorption and emission (706 and 747 nm, respectively) were observed in 3,5-(5-methylthiophene)benzoBODIPY 1e in toluene. Electronic properties of the phenyl substituents also affected their absorption and emission properties. BenzoBODIPY 1a possessing strong electron-donating methoxy group at the para-position of 3,5-phenyl groups showed the longer absorption and emission maximum (658 nm and 690, respectively) relative to other 3,5-diphenylbenzoBODIPYs 1b–d in toluene.| BenzoBODIPYs | λmaxabs (nm) | λmaxem (nm) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) εmax εmax |
Φa | Stokes shift (cm−1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a The fluorescence quantum yields were calculated using ZnPc in DMF solution (Φ = 0.28) as reference for 1a–d and 1,3,5,7-(4-methoxy)phenylazaBODIPY (Φ = 0.36 in chloroform) for 1e, respectively. The standard errors are less than 5%. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1a | 658 | 690 | 4.84 | 0.70 | 705 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1b | 653 | 681 | 5.04 | 0.76 | 630 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1c | 646 | 674 | 4.94 | 0.78 | 643 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1d | 648 | 679 | 5.01 | 0.75 | 704 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1e | 706 | 747 | 4.86 | 0.35 | 777 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Increase of the polarity of solvents from toluene to dichloromethane, THF, methanol and acetonitrile led to 12–16 and 10–11 nm blue-shift of the absorption and emission maximum, respectively (Table S3, ESI†). All these benzoBODIPYs except 1e showed intense fluorescence with the fluorescence quantum yields between 0.70 and 0.99 in all the solvents investigated. These values were higher than that of their non-fused BODIPY analogs.28 The relatively low fluorescence quantum yields (0.30–0.36) observed in 1e may associated with the rotation of thiophene moieties and the heavy atom effect of sulfur atoms.29 Only a slight variation of the fluorescence quantum yields were observed for these benzoBODIPYs with the variation of the solvent polarity. This may closely associated with their rigid structures. Our result is in good consistence with literature reported aromatic-ring-fused π-extended chromophores.22
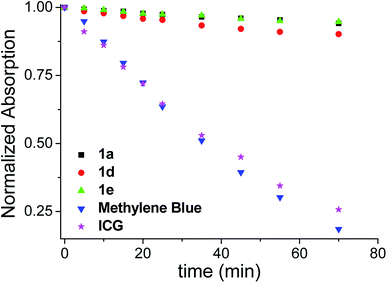
These benzoBODIPYs showed excellent photostabilities as demonstrated by 1a, 1d and 1e in toluene in Fig. 4. During the period (70 min) of strong irradiation with a 500 W Xe lamp, more than 90% of 1a, 1d and 1e remained, while only 26% of the well-known commercialized Indocyanine Green (ICG) and 18% of methylene blue were left under this condition.
DFT calculation
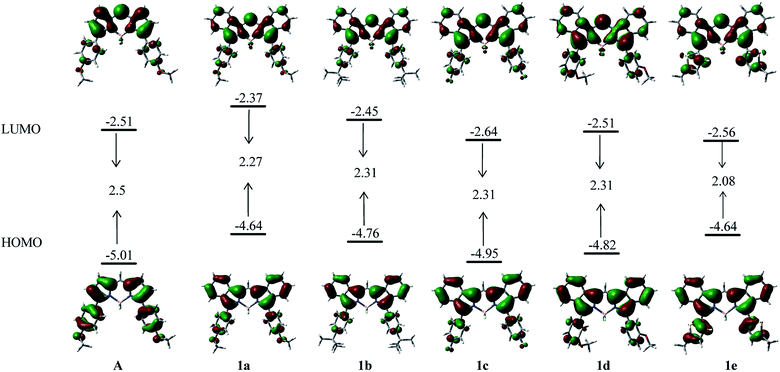
For a better understanding of the optical properties of these benzoBODIPYs, their transitional energies were calculated using time-dependent density functional theory (TD-DFT) at the B3LYP/6-31G(d,p) level (Table S4, ESI†). Non-fused 3,5-diarylBODIPY A as an analogue of 1a was also investigated and was used as a reference compound for comparison. The calculated absorption maximum was in good agreement with the absorption spectra of these benzoBODIPYs in dichloromethane. The longest absorption maximum for all these benzoBODIPYs were mainly contributed by the HOMO–LUMO transition (Table S4, ESI†). In comparison with reference compound A, benzene-annulation resulted in the increased HOMO (0.37 eV) and LUMO (0.14 eV) energies and the overall decrease of the bandgap (0.23 eV) for 1a (Fig. 5). Electron-donating substituents on 3,5-phenyls destabilize both HOMO and LUMO. For example, the HOMO/LUMO energies decreased from −4.95/−2.64 eV (1c) to −4.76/−2.45 (1b) and −4.64/−2.37 eV (1a). Interestingly, in comparison with 1a, 1e showed a decreased LUMO level from −2.37 to −2.56 eV with the HOMO energy level remained unchanged (Fig. 5). This led to a decreased HOMO–LUMO energy gap (0.19 eV), and is in good agreement with a 48 nm further red-shift in the absorption spectra in toluene relative to 1a.As shown in Fig. 5, a stronger electron-density distribution on the annulated benzene moieties was found in the HOMO orbitals in comparison to that in the LUMO orbitals. This is in agreement with that benzene annulation (1a) largely increased the HOMO energy level with respect to non-fused A. Both HOMO and LUMO are also localized on the 3,5-aryl groups in these benzoBODIPYs. Interestingly, a stronger HOMO and LUMO delocalization on the 5-methylthiophene units was observed in 1e relative to those on phenyls in 1a–d. This may indicate thiophene moieties in 1e provide a better conjugation with the benzoBODIPY chromophore than that of phenyl moieties in 1a–d.
Conclusion
In conclusion, a set of photostable 3,5-diarylated benzoBODIPYs with strong absorption and emission in the far-red to NIR region (λmaxabs ≤ 706 nm, λmaxem ≤ 745 nm) were synthesized within three steps from commercial isoindolin-1-one. In comparison with the non-fused BODIPY analogue, these benzoBODIPYs showed a more than 80 nm red-shift in both absorption and emission maximum and an enhanced fluorescence. Their optical properties can be easily modulated via the variation of the electronic properties of 3,5-diaryl moieties. DFT calculations indicated the localization of HOMO and LUMO at the two annulated benzene moieties and the 3,5-aryl groups. The electron-donating groups on the 3,5-aryl groups caused a destabilized HOMO and LUMO, while the 3,5-thiophenes induced an increased HOMO and a decreased LUMO, and a better conjugation with benzoBODIPY chromophore than that of phenyl moieties.Experimental section
General
Reagents and solvents were used as received from commercial suppliers unless noted otherwise. All reactions were performed in oven-dried or flame-dried glassware unless otherwise stated, and were monitored by TLC using 0.25 mm silica gel plates with UV indicator (60F-254). 1H and 13C NMR were recorded on 300 MHz or 500 MHz NMR spectrometers at room temperature. Chemical shifts (δ) are given in ppm relative to CDCl3 (7.26 ppm for 1H and 77 ppm for 13C) or to internal TMS.30 High-resolution mass spectra (HRMS) were obtained using APCI-TOF or MALDI-TOF in positive mode. Photostabilities were studied by continuous irradiation with a Xe lamp (500 W) in DMF.UV-visible absorption spectra and fluorescence emission spectra were recorded on a commercial spectrophotometer (Shimadzu UV-2450 and Hitachi F-4500) at room temperature. Relative fluorescence quantum efficiencies of dyes were obtained by comparing the areas under the corrected emission spectrum of the test sample in various solvents with reference compound. Fluorescence quantum yields were calculated using ZnPc (ϕ = 0.28, in DMF)31a and 1,7-diphenyl-3,5-di(4-methoxyphenyl)-azadipyrromethene (ϕ = 0.36 in chloroform).31b Non-degassed, spectroscopic grade solvents and a 10 mm quartz cuvette were used. Dilute solutions (0.01 < A < 0.05) were used to minimize the reabsorption effects. Quantum yields were determined using the following equation:32
The subscripts x and r refer respectively to our sample x and reference (standard) fluorophore r with known quantum yield Φr in a specific solvent, F stands for the spectrally corrected, integrated fluorescence spectra, A(λex) denotes the absorbance at the used excitation wavelength λex, and n represents the refractive index of the solvent (in principle at the average emission wavelength).
Crystals of dyes 1a (CCDC 1445553), 1c (CCDC 1445552) and 1d (CCDC 1445551)† suitable for X-ray analysis were obtained by slow diffusion of methanol into their dichloromethane solutions at room temperature. A suitable crystal was chosen and mounted on a glass fiber using grease. Data were collected using a diffractometer equipped with a graphite crystal monochromator situated in the incident beam for data collection at room temperature. Cell parameters were retrieved using SMART33 software and refined using SAINT34 on all observed reflections. The determination of unit cell parameters and data collections were performed with Mo Kα radiation (λ) at 0.71073 Å. Data reduction was performed using the SAINT software, which corrects for Lp and decay. The structure was solved by the direct method using the SHELXS-974 program and refined by least squares method on F2, SHELXL-97,35 incorporated in SHELXTL V5.10.36
Computational details
The ground state geometry was optimized by using DFT method at B3LYP/6-31G (d) level. The same method was used for vibrational analysis to verify that the optimized structures correspond to local minima on the energy surface. TD-DFT computations were used to obtain the vertical excitation energies and oscillator strengths at the optimized ground state equilibrium geometries under the B3LYP/6-31+G(d,p) theoretical level. TD-DFT calculated molecules in dichloromethane were done using the Self-Consistent Reaction Field (SCRF) method and Polarizable Continuum Model (PCM). All of the calculations were carried out by the methods implemented in Gaussian 09 package.37Synthesis
Compound 3 was synthesized according to literatures.24,26Compound 2a. Compound 2a was prepared in 72% yield (289 mg) from 3 (400 mg, 1.6 mmol) and 4-methoxyphenylboronic acid (532 mg, 3.5 mmol). 1H NMR (300 MHz, CDCl3) δ 9.87 (s, 1H), 7.98 (d, J = 6.0 Hz, 2H), 7.77 (d, J = 6.0 Hz, 2H), 7.41 (br, 1H), 7.26 (br, 1H), 7.09 (d, J = 9.0 Hz, 2H), 3.90 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 173.0, 160.4, 135.7, 133.2, 129.5, 127.4, 123.9, 123.5, 123.0, 122.1, 121.8, 117.4, 114.6, 55.4. HRMS (ESI) calcd for C16H13NO2 [M + H]+: 252.1025, found 252.1019.
Compound 2b. Compound 2b was prepared in 73% yield (323 mg) from 3 (400 mg, 1.6 mmol) and 4-tert-butylphenylboronic acid (623 mg, 3.5 mmol). 1H NMR (300 MHz, CDCl3) δ 9.90 (s, 1H), 8.04–7.98 (m, 2H), 7.78 (d, J = 6.0 Hz, 2H), 7.58 (d, J = 9.0 Hz, 2H), 7.41 (t, J = 7.5 Hz, 1H), 7.24 (t, J = 6.0 Hz, 1H), 1.39 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 173.6, 152.5, 134.4, 132.4, 127.7, 127.4, 127.3, 126.4, 123.9, 123.7, 122.1, 121.9, 117.5, 34.9, 31.2. HRMS (ESI) calcd for C19H19NO [M + H]+: 278.1545, found 278.1539.
Compound 2c. Compound 2c was prepared in 53% yield (202 mg) from 3 (400 mg, 1.6 mmol) and 4-fluorophenylboronic acid (490 mg, 3.5 mmol). 1H NMR (300 MHz, CDCl3) δ 9.91 (s, 1H), 8.02–7.93 (m, 2H), 7.83 (brs, 2H), 7.43 (t, J = 7.5 Hz, 1H), 7.29–7, 24 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 174.3, 164.6, 162.6, 133.3, 132.5, 129.9, 127.9, 127.2, 124.4, 124.2, 122.5, 121.9, 118.0, 116.9. F–C coupling was not observed due to the limited solubility. HRMS (ESI) calcd for C15H10FNO [M + H]+: 240.0825, found 240.0819.
Compound 2d. Compound 2d was prepared in 70% yield (281 mg) from 3 (400 mg, 1.6 mmol) and 3-methoxyphenylboronic acid (532 mg, 3.5 mmol). 1H NMR (300 MHz, CDCl3) δ 12.08 (s, 1H), 9.89 (s, 1H), 8.02–7.97 (m, 4H), 7.43 (brs, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.00 (brs, 1H), 3.90 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 173.7, 160.2, 134.8, 132.7, 131.7, 130.3, 127.5, 124.1, 123.9, 122.0, 120.4, 117.5, 115.2, 113.0, 55.5. HRMS (ESI) calcd for C16H13NO2 [M + H]+: 252.1025, found 252.1019.
Compound 2e. Compound 2e was prepared in 65% yield (236 mg) from 3 (400 mg, 1.6 mmol) and 5-methylthiophene-2-boronic acid (497 mg, 3.5 mmol). 1H NMR (300 MHz, CDCl3) δ 9.86 (s, 1H), 8.07 (d, J = 9.0 Hz, 1H), 7.97 (d, J = 9.0 Hz, 1H), 7.55 (d, J = 3.0 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.29–7.24 (m, 1H), 6.88 (d, J = 3.0 Hz, 1H), 2.59 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 171.9, 141.1, 131.2, 128.9, 127.9, 126.6, 125.6, 125.5, 122.9, 122.6, 121.0, 120.5, 116.5, 14.4. HRMS (APCI) calcd for C14H12NOS [M + H]+: 242.0640, found 242.0634.
Compound 1a. Compound 1a was prepared in 64% yield (161 mg) from 2a (254 mg, 1.0 mmol). 1H NMR (500 MHz, CDCl3) δ 7.79 (brs, 6H), 7.63 (brs, 2H), 7.44 (t, J = 7.5 Hz, 3H), 7.26–7.23 (m, 3H), 7.04 (d, J = 5.0 Hz, 4H), 3.88 (s, 6H). 13C NMR (75 MHz, CDCl3): δ 160.6, 151.3, 134.2, 131.8, 130.4, 128.8, 127.5, 125.1, 123.7, 123.6, 118.9, 113.9, 113.5, 55.3. HRMS (APCI) calcd for C31H24O2N2BF2 [M + H]+: 505.1899, found 505.1898.
Compound 1b. Compound 1b was prepared in 73% yield (202 mg) from 2b (278 mg, 1.0 mmol). 1H NMR (300 MHz, CDCl3) δ 7.91 (d, J = 6.0 Hz, 4H), 7.80 (d, J = 9.0 Hz, 4H), 7.68 (d, J = 9.0 Hz, 2H), 7.53–7.44 (m, 7H), 1.38 (s, 18H). 13C NMR (75 MHz, CDCl3): δ 152.5, 151.8, 134.2, 130.7, 130.0, 128.9, 128.2, 127.6, 125.3, 125.1, 123.9, 118.9, 114.3, 34.9, 31.3. HRMS (APCI) calcd for C37H36N2BF2 [M + H]+: 557.2940, found 557.2944.
Compound 1c. Compound 1c was prepared in 41% yield (98 mg) from 2c (120 mg, 0.5 mmol). 1H NMR (300 MHz, CDCl3) δ 7.93 (d, J = 9.0 Hz, 6H), 7.86–7.79 (m, 4H), 7.61 (d, J = 9.0 Hz, 2H), 7.49 (t, J = 7.5 Hz, 2H), 7.30–7.17 (m, 7H). 13C NMR (125 MHz, CDCl3) δ 165.0, 163.0, 151.1, 134.6, 132.7, 131.0, 129.6, 128.0, 127.4, 125.9, 123.8, 119.4, 116.0, 115.9. F–C couping was not observed due to its limited solubility. HRMS (APCI) calcd for C29H18N2BF4 [M + H]+: 481.1499, found 481.1494.
Compound 1d. Compound 1d was prepared in 69% yield (174 mg) from 2d (254 mg, 1.0 mmol). 1H NMR (300 MHz, CDCl3) δ 7.91 (brs, 4H), 7.69 (brs, 2H), 7.44–7.40 (m, 9H), 7.02 (brs, 2H), 3.85 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 159.2, 151.6, 134.3, 132.2, 130.5, 129.2, 129.1, 127.7, 125.3, 123.7, 122.7, 118.9, 115.8, 115.4, 110.0, 55.3. HRMS (APCI) calcd for C31H24O2N2BF2 [M + H]+: 505.1899, found 505.1895.
Compound 1e. Compound 1e was prepared in 46% yield (111 mg) from 2f (120 mg, 0.5 mmol). 1H NMR (300 MHz, CDCl3) δ 8.06 (d, J = 7.5 Hz, 2H), 7.89–7.86 (m, 4H), 7.69 (brs, 1H), 7.45 (t, J = 7.5 Hz, 2H), 7.32–7.30 (m, 2H), 6.95 (d, J = 3.0 Hz, 2H), 2.61 (s, 6H). 13C NMR was not available due to poor solubility. HRMS (MALDI-TOF) calcd for C27H20N2S2BF2 [M + H]+: 485.1129, found 485.1198.
Acknowledgements
This work is supported by the National Nature Science Foundation of China (Grants No. 21372011, 21402001 and 21472002) and Nature Science Foundation of Anhui Province (Grants No. 1508085J07). The numerical calculations in this paper have been done on the supercomputing system in the Supercomputing Center of University of Science and Technology of China.References
- (a) K. Y. Law, Chem. Rev., 1993, 93, 449 CrossRef CAS; (b) V. J. Pansare, S. Hejazi, W. J. Faenza and R. K. Prud’homme, Chem. Mater., 2012, 24, 812 CrossRef CAS PubMed; (c) G. Qian and Z. Y. Wang, Chem.–Asian J., 2010, 5, 1006 CrossRef CAS PubMed; (d) Y. Yang, M. Lowry, X. Xu, J. O. Escobedo, M. Sibrian-Vazquez, L. Wong, C. M. Schowalter, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8829 CrossRef CAS PubMed; (e) V. J. Pansare, S. Hejazi, W. J. Faenza and R. K. Prud’homme, Chem. Mater., 2012, 24, 812 CrossRef CAS PubMed.
- (a) L. Dou, Y. Liu, Z. Hong, G. Li and Y. Yang, Chem. Rev., 2015, 115, 12633 CrossRef CAS PubMed; (b) B. M. Squeo, N. Gasparini, T. Ameri, A. Palma-Cando, S. Allard, V. G. Gregoriou, C. J. Brabec, U. Scherf and C. L. Chochos, J. Mater. Chem. A, 2015, 3, 16279 RSC; (c) X. Zhang, Y. Zhang, L. Chen and Y. Xiao, RSC Adv., 2015, 5, 32283 RSC; (d) M. E. Ragoussi and T. Torres, Chem. Commun., 2015, 51, 3957 RSC.
- (a) L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622 RSC; (b) Y. Ding, Y. Tang, W. Zhu and Y. Xie, Chem. Soc. Rev., 2015, 44, 1101 RSC; (c) J. Fan, M. Hu, P. Zhan and X. Peng, Chem. Soc. Rev., 2013, 42, 29 RSC; (d) K. Umezawa, D. Citterio and K. Suzuki, Anal. Sci., 2014, 30, 327 CrossRef CAS PubMed; (e) M. Ptaszek, Prog. Mol. Biol. Transl. Sci., 2013, 113, 59 CAS; (f) S. Luo, E. Zhang, Y. Su, T. Cheng and C. Shi, Biomaterials, 2011, 32, 712 Search PubMed; (g) Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16 RSC.
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184 CrossRef CAS PubMed; (c) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496 RSC.
- (a) A. Bessette and G. S. Hanan, Chem. Soc. Rev., 2013, 43, 3347 Search PubMed; (b) T. Bura, N. Leclerc, S. Fall, P. Lévêque, T. Heiser, P. Retailleau, S. Rihn, A. Mirloup and R. Ziessel, J. Am. Chem. Soc., 2012, 134, 17404 CrossRef CAS PubMed; (c) J. J. Chen, S. M. Conron, P. Erwin, M. Dimitriou, K. McAlahney and M. E. Thompson, ACS Appl. Mater. Interfaces, 2015, 7, 662 CrossRef CAS PubMed; (d) W. Liu, A. Tang, J. Chen, Y. Wu, C. Zhan and J. Yao, ACS Appl. Mater. Interfaces, 2014, 6, 22496 CrossRef CAS PubMed.
- (a) Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16 RSC; (b) S.-W. Yun, N.-Y. Kang, S.-J. Park, H.-H. Ha, Y. K. Kim, J.-S. Lee and Y.-T. Chang, Acc. Chem. Res., 2014, 47, 1277 CrossRef CAS PubMed; (c) X. Zhang, Y. Xiao, J. Qi, J. Qu, B. Kim, X. Yue and K. D. Belfield, J. Org. Chem., 2013, 78, 9153 CrossRef CAS PubMed.
- (a) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130 RSC; (b) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953 RSC; (c) M. vedamalai, D. Kedaria, R. Vasita, S. Mori and I. Gupta, Dalton Trans., 2016, 45, 2700 RSC; (d) B. Umasekhar, E. Ganapathi, T. Chatterjee and M. Ravikanth, Dalton Trans., 2015, 44, 16516 RSC.
- (a) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77 RSC; (b) A. G. Awuah and Y. You, RSC Adv., 2012, 2, 11169 RSC; (c) J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323 RSC; (d) Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937 CrossRef CAS PubMed.
- N. Boens, B. Verbelen and W. Dehaen, Eur. J. Org. Chem., 2015, 6577 CrossRef CAS.
- (a) H. Lu, J. Mack, Y. Yang and Z. Shen, Chem. Soc. Rev., 2014, 43, 4778 RSC; (b) Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774 RSC.
- (a) S. Atilgan, Z. Ekmekci, A. L. Dogan, D. Guc and E. U. Akkaya, Chem. Commun., 2006, 4398 RSC; (b) S. Ozlem and E. U. Akkaya, J. Am. Chem. Soc., 2009, 131, 48 CrossRef CAS PubMed; (c) S. Erbas-Cakmak and E. U. Akkaya, Org. Lett., 2014, 16, 2946 CrossRef CAS PubMed; (d) H. He, P.-C. Lo, S.-L. Yeung, W.-P. Fong and D. K. P. Ng, Chem. Commun., 2011, 47, 4748 RSC.
- (a) V. Leen, M. Van der Auweraer, N. Boens and W. Dehaen, Org. Lett., 2011, 13, 1470 CrossRef CAS PubMed; (b) V. Leen, F. Schevenels, J. Cui, C. Xu, W. Yang, X. Tang, W. Liu, W. Qin, W. M. De Borggraeve, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2009, 5920 CrossRef CAS; (c) L. Jiao, C. Yu, T. Uppal, M. Liu, Y. Li, Y. Zhou, E. Hao, X. Hu and M. G. H. Vicente, Org. Biomol. Chem., 2010, 8, 2517 RSC; (d) H. Wang, F. R. Fronczek, M. G. H. Vicente and K. M. Smith, J. Org. Chem., 2014, 79, 10342 CrossRef CAS PubMed.
- (a) S. L. Niu, G. Ulrich, R. Ziessel, A. Kiss, P.-Y. Renard and A. Romieu, Org. Lett., 2009, 11, 2049 CrossRef CAS PubMed; (b) S. Zhu, J. Zhang, G. Vegesna, F.-T. Luo, S. A. Green and H. Liu, Org. Lett., 2011, 13, 438 CrossRef CAS PubMed; (c) A. Poirel, A. De Nicola and R. Ziessel, J. Org. Chem., 2014, 79, 11463 CrossRef CAS PubMed; (d) H. Yokoi, S. Hiroto and H. Shinokubo, Org. Lett., 2014, 16, 3004 CrossRef CAS PubMed; (e) B. Dhokale, T. Jadhav, S. M. Mobin and R. Misra, Dalton Trans., 2016, 45, 1476 RSC; (f) B. Dhokale, T. Jadhav, S. M. Mobin and R. Misra, Dalton Trans., 2015, 44, 15803 RSC.
- (a) A. B. Descalzo, H. Xu, Z. Xue, K. Hoffmann, Z. Shen, M. G. Weller, X. You and K. Rurack, Org. Lett., 2008, 10, 1581 CrossRef CAS PubMed; (b) Y. Hayashi, N. Obata, M. Tamaru, S. Yamaguchi, Y. Matsuo, A. Saeki, S. Seki, Y. Kureishi, S. Shohei, S. Yamaguchi and H. Shinokubo, Org. Lett., 2012, 14, 866 CrossRef CAS PubMed.
- (a) L. Luo, D. Wu, W. Li, S. Zhang, Y. Ma, S. Yuan and J. You, Org. Lett., 2014, 16, 6080 CrossRef CAS PubMed; (b) X. Zhou, Q. Wu, Y. Feng, Y. Yu, C. Yu, E. Hao, Y. Wei, X. Mu and L. Jiao, Chem.–Asian J., 2015, 10, 1979 CrossRef CAS PubMed.
- (a) K. Umezawa, Y. Nakamura, H. Makino, D. Citterio and K. Suzuki, J. Am. Chem. Soc., 2008, 130, 1550 CrossRef CAS PubMed; (b) K. Umezawa, A. Matsui, Y. Nakamura, D. Citterio and K. Suzuki, Chem.–Eur. J., 2009, 15, 1096 CrossRef CAS PubMed; (c) V. Leen, W. Qin, W. Yang, J. Cui, C. Xu, X. Tang, W. Liu, K. Robeyns, L. V. Meervelt, D. Beljome, R. Lazzaroni, C. Tonnele, N. Boens and W. Dehaen, Chem.–Asian J., 2010, 5, 2016 CrossRef CAS PubMed; (d) J. Chen, A. Burghart, A. Derecskei-Kovacs and K. Burgess, J. Org. Chem., 2000, 65, 2900 CrossRef CAS PubMed.
- (a) S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884 CrossRef CAS PubMed; (b) Y. Yang, Q. Guo, H. Chen, Z. Zhou, Z. Guo and Z. Shen, Chem. Commun., 2013, 49, 3940 RSC; (c) S. G. Awuah, S. K. Das, F. D'Souza and Y. You, Chem.–Asian J., 2013, 8, 3123 CrossRef CAS PubMed; (d) K. Tanaka, H. Yamane, R. Yoshii and Y. Chujo, Bioorg. Med. Chem., 2013, 21, 2715 CrossRef CAS PubMed; (e) R. L. Watley, S. G. Awuah, M. Bio, R. Cantu, H. B. Gobeze, V. N. Nesterov, S. K. Das, F. D'Souza and Y. You, Chem.–Asian J., 2015, 10, 1335 CrossRef CAS PubMed; (f) S. Ji, J. Ge, D. Escudero, Z. Wang, J. Zhao and D. Jacquemin, J. Org. Chem., 2015, 80, 5958 CrossRef CAS PubMed; (g) Z. Sun, M. Guo and C. Zhao, J. Org. Chem., 2016, 81, 229 CrossRef CAS PubMed.
- (a) C. Jiao, L. Zhu and J. Wu, Chem.–Eur. J., 2011, 17, 6610 CrossRef CAS PubMed; (b) C. Jiao, K. Huang and J. Wu, Org. Lett., 2011, 13, 632 CrossRef CAS PubMed; (c) L. Zeng, C. Jiao, X. Huang, K. Huang, W. Chin and J. Wu, Org. Lett., 2011, 13, 6026 CrossRef CAS PubMed; (d) M. H. Chua, K. Huang, J. Xu and J. Wu, Org. Lett., 2015, 17, 4168 CrossRef CAS PubMed; (e) E. Heyer, P. Retailleau and R. Ziessel, Org. Lett., 2014, 16, 2330 CrossRef CAS PubMed.
- (a) V. F. Donyagina, S. Shimizu, N. Kobayashi and E. A. Lukyanets, Tetrahedron Lett., 2008, 49, 6152 CrossRef CAS; (b) H. Lu, S. Shimizu, J. Mack, Z. Shen and N. Kobayashi, Chem.–Asian J., 2011, 6, 1026 CrossRef CAS PubMed; (c) H. Liu, J. Mack, Q. Fuo, H. Lu, N. Kobayashi and Z. Shen, Chem. Commun., 2011, 47, 12092 RSC; (d) R. Gresser, M. Hummert, H. Hartmann, K. Leo and M. Riede, Chem.–Eur. J., 2011, 17, 2939 CrossRef CAS PubMed; (e) P. Majumdar, J. Mack and T. Nyokong, RSC Adv., 2015, 5, 78253 RSC; (f) A. Diaz -Moscoso, E. Emond, D. L. Hughes, G. J. Tizzard, S. J. Coles and A. N. Cammidge, J. Org. Chem., 2014, 79, 8932 CrossRef CAS PubMed; (g) E. Magligaspe, T. J. Pundsack, L. M. Albert, Y. V. Zatsikha, P. V. Solntsev, D. A. Blank and V. N. Nemykin, Inorg. Chem., 2015, 54, 4167 CrossRef PubMed.
- (a) A. Wakamiya, T. Murakami and S. Yamaguchi, Chem. Sci., 2013, 4, 1002 RSC; (b) Y. Ni, W. Zeng, K. Huang and J. Wu, Chem. Commun., 2013, 49, 1217 RSC; (c) H. Shimogawa, H. Mori, A. Wakamiya and Y. Murata, Chem. Lett., 2013, 42, 986 CrossRef CAS.
- (a) Z. Shen, H. Röhr, K. Rurack, H. Uno, M. Spieles, B. Schulz, G. Reck and N. Ono, Chem.–Eur. J., 2004, 10, 4853 CrossRef CAS PubMed; (b) Y. Tomimori, T. Okujima, T. Yano, S. Mori, N. Ono, H. Yamada and H. Uno, Tetrahedron, 2011, 67, 3187 CrossRef CAS; (c) M. Nakamura, H. Tahara, K. Takahashi, T. Nagata, H. Uoyama, D. Kuzuhara, S. Mori, T. Okujima, H. Yamada and H. Uno, Org. Biomol. Chem., 2012, 10, 6840 RSC; (d) M. Nakamura, M. Kitatsuka, K. Takahashi, T. Nagata, S. Mori, D. Kuzuhara, T. Okujima, H. Yamada, T. Nakae and H. Uno, Org. Biomol. Chem., 2014, 12, 1309 RSC; (e) T. Aotake, M. Suzuki, K. Tahara, N. Aratani, N. Tamai and H. Yanada, Chem.–Eur. J., 2015, 21, 4966 CrossRef CAS PubMed.
- (a) T. Uppal, X. Hu, F. R. Fronczek, S. Maschek, P. Bobadova-Parvanova and M. G. H. Vicente, Chem.–Eur. J., 2012, 18, 3893 CrossRef CAS PubMed; (b) M. A. Filatov, A. Y. Lebedev, S. N. Mukhin, S. A. Vinogradov and A. V. Cheprakov, J. Am. Chem. Soc., 2010, 132, 9552 CrossRef CAS PubMed; (c) Q. Meng, F. R. Fronczeka and M. G. H. Vicente, New J. Chem., 2016 10.1039/c5nj03324a.
- (a) G. Ulrich, S. Goeb, A. De Nicola, P. Retailleau and R. Ziessel, J. Org. Chem., 2011, 76, 4489 CrossRef CAS PubMed; (b) Y. Suda, R. Nishiyabu and Y. Kubo, Tetrahedron, 2015, 71, 4174 CrossRef CAS; (c) A. Nagai and Y. Chujo, Macromolecules, 2010, 43, 193 CrossRef CAS; (d) S. Yamazawa, M. Nakashima, Y. Suda, R. Nishiyabu and Y. Kubo, J. Org. Chem., 2016, 81, 1310 CrossRef CAS PubMed.
- (a) C. Yu, Y. Xu, L. Jiao, J. Zhou, Z. Wang and E. Hao, Chem.–Eur. J., 2012, 18, 6437 CrossRef CAS PubMed; (b) L. Jiao, C. Yu, M. Liu, Y. Wu, K. Cong, T. Meng, Y. Wang and E. Hao, J. Org. Chem., 2010, 75, 6035 CrossRef CAS PubMed; (c) E. Hao, Y. Xu, C. Yu and L. Jiao, CN103952001A, 2014.
- L. Wu and K. Burgess, Chem. Commun., 2008, 40, 4933 RSC.
- P. Diana, A. Martorana, P. Barraja, A. Montalbano, A. Carbone and G. Cirrincione, Tetrahedron, 2011, 67, 2072 CrossRef CAS.
- (a) K. Rurack, M. Kollamannasberger and J. Daub, Angew. Chem., Int. Ed., 2001, 40, 385 CrossRef CAS; (b) S. Ozlem and E. U. Akkaya, J. Am. Chem. Soc., 2009, 131, 48 CrossRef CAS PubMed; (c) O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644 CrossRef CAS PubMed; (d) T. Bura, P. Retaileau, G. Ulrich and R. Ziessel, J. Org. Chem., 2011, 76, 1109 CrossRef CAS PubMed; (e) F. Ali, H. A. Anila, N. Taye, R. G. Gonnade, S. Chattopadhyay and A. Das, Chem. Commun., 2015, 51, 16932 RSC.
- B. Verbelenk, S. Boodts, J. Hofkens, N. Boens and W. Dehaen, Angew. Chem., Int. Ed., 2015, 54, 4612 CrossRef PubMed.
- (a) A. Poirel, A. De Nicola, P. Retailleau and R. Ziessel, J. Org. Chem., 2012, 77, 7512 CrossRef CAS PubMed; (b) A. Poirel, A. De Nicola and R. Ziessel, Org. Lett., 2012, 14, 5696 CrossRef CAS PubMed.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176 CrossRef CAS.
- (a) I. Scalise and E. N. Durantini, Bioorg. Med. Chem., 2005, 13, 3037 CrossRef CAS PubMed; (b) A. Gorman, J. Killoran, C. O’ Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619 CrossRef CAS PubMed.
- J. Lakowicz, Principles of Fluorescence Spectroscopy, Springer-Verlag, New York, 3rd edn, 2006 Search PubMed.
- SAINT V 6.01 (NT) Software for the CCD Detector System, Bruker Analytical X-ray Systems, Madison, WI, 1999 Search PubMed.
- G. M. Sheldrick, SHELXS-90, Program for the Solution of Crystal Structure, University of Göttingen, Germany, 1990 Search PubMed.
- SHELXL-97, Program for the Refinement of Crystal Structure, University of Göttingen, Germany, 1997 Search PubMed.
- SHELXTL 5.10 (PC/NT-Version), Program library for StructureSolution and Molecular Graphics, Bruker Analytical X-ray Systems, Madison, WI, 1998 Search PubMed.
- M. J. Frisch, G. W. Trucks and H. B. Schlegel, et al., Gaussian 09, Revision B.01, Gaussian Inc, Wallingford CT, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal structure determination and CIF files, additional photophysical data and spectra, copies of NMR spectra and high resolution mass spectra for all new compounds, calculated frontier orbitals and absorption spectra. CCDC 1445551–1445553. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra04412c |
| This journal is © The Royal Society of Chemistry 2016 |