Formation of onion-like fullerene and chemically converted graphene-like nanosheets from low-quality coals: application in photocatalytic degradation of 2-nitrophenol†
Tonkeswar Dasa,
Purna K. Boruahbc,
Manash R. Dasbc and
Binoy K. Saikia*ac
aCoal Chemistry Division, CSIR-North East Institute of Science & Technology, Jorhat-785006, India. E-mail: bksaikia@gmail.com; bksaikia@rrljorhat.res.in; Tel: +91 376 2372581
bMaterials Science Division, CSIR-North East Institute of Science & Technology, Jorhat-785006, India
cAcademy of Scientific and Innovative Research, CSIR-NEIST Campus, Jorhat-785006, India
First published on 4th April 2016
Abstract
The formation of coal-derived carbon nanomaterials (CNMs) consisting of onion-like fullerene and chemically converted graphene-like nanosheets from the low-quality coals were observed during an oxidation-cum-extraction (OCE) process. Detailed characterization of these CNMs was examined by using scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), high resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), Raman spectroscopy, Fourier transform infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), solid-state 13C NMR, UV-visible spectroscopy, and fluorescence spectroscopy (FL) techniques. The size of the coal-derived onion-like fullerenes was found to be in the size range of 5–20 nm and composed of 5–20 nm concentric shells. The outer diameter of the onion-like structures was estimated to be in the range of 3–15 nm. The presence of clusters of chemically converted graphene-like nanosheets was also observed in the coal-derived CNMs obtained by this novel OCE process. The method reported in this paper could be an inexpensive and simple chemical process for the preparation of CNMs from coals. These low-quality coal-derived CNMs were utilized as an efficient photocatalyst for the degradation of hazardous 2-nitrophenol under natural sunlight irradiation.
1. Introduction
Coal is being considered as a feedstock for carbon-based materials such as carbon nanomaterials, which include carbon nanotubes, fullerenes, carbon nanodots, nanofibers, nanodiamonds, graphene nanosheets, graphene onions. These materials have generated significant attention among researchers since their discovery due to their unique optical, electrical, thermal, and mechanical properties.1–4 The worldwide current market price for various types of carbon nanomaterials depends upon their purity with cost increasing as purity increases. Large quantities of various types of carbon nanomaterials are needed in the market-driven applications, such as composites, catalyst support, semiconductors, electronics, bio sensing, bio imaging, drug delivery and environmental applications. High costs could drastically decrease the benefits of using carbon nanomaterials in these applications; hence there is a substantial demand for demonstrating practical techniques for the selective large-scale synthesis of high purity carbon nanomaterials from easily available and cheap materials such as coal. If the carbon nanomaterials from coal could be obtained by a simple typical less-expensive method, then coal could be a good substitute for the conventional materials, as they are easily and economically available natural carbon resource.Several researchers have published descriptions for the synthesis of carbon-based nanomaterials from coals by using different methods.5–25 However, it is to be noted that all those existing methods involve high temperatures, high energy, high cost, and drastic treatments to the coals and none of the methods reported are simple and an inexpensive way for large scale production of carbon nanomaterials from coal. This deficiency has recently motivated researchers to investigate simple treatments such as chemical oxidation methods for the production of the carbon nanomaterials.4,26–29
In the present manuscript a description is given for the process of the formation of onion-like fullerenes and chemically converted graphene-like nanosheets from low-quality coals of the Northeast region of India (NER) via a simple chemical technique, which is different from the chemical methods, reported previously,4,26–29 which could be used amongst others for water cleaning processes.
Recently, the use of carbon nanomaterials as novel material has found tremendous attention in removal of organic pollutants from waste water, which has become one of the most serious global environmental issues of the 21st century.30,31 Nitrophenols are recognized as one of the major organic pollutant of water systems due to their high toxicity32,33 and are generally used in pharmaceutical, fertilizer or dye industries.32,33 It is difficult to purify nitrophenol-contaminated wastewater due to their resistance to chemical and biological degradation.34,35 Therefore, the development of a stable material and a cost-effective technique to reduce the concentration of nitrophenol along with the other organic pollutants is becoming a challenging task in the different industries. In the present investigation, the proper utilization of the formed coal-derived carbon nanomaterials (CNMs) for removal of hazardous nitrophenol from aqueous solutions was also targeted.
2. Experimental sections
2.1 Materials
The raw coal samples (Coal-NG and Coal-NK) were from the Northeastern coalfields (India). The coal samples (∼1 kg each) were first air dried, crushed to below 3.180 mm and finally sieved to a <0.211 mm size using standard methods.36 Analytical grade (AR) chemicals, such as nitric acid (HNO3) (95–98%, Merck), sodium hydroxide (NaOH) (Merck) and hydrochloric acid (HCl) (Merck), and 2-nitrophenol (Alfa Aesar) were commercially purchased and used as received.2.2 Oxidation followed by alkaline extraction of coal
In a typical procedure, approximately 50 g of each coal sample (0.211 mm fineness) was carefully mixed with 200 mL HNO3 (98%). The mixture was stirred and heated in an oil bath at 150 °C for 2 h at atmospheric pressure. The mixture was then cooled, filtered, and washed with a 1 N HCl solution followed by de-ionized water until the pH of the filtrate become neutral. The residue was recovered, dried, and collected as oxidized coal.40 g of each oxidized coal samples was mixed with 400 mL 1 N NaOH in a beaker and continuously stirred for 24 h with a gel-like blackish colored solution resulting. The solid residue in the solution was separated by centrifugation and the supernatants were collected. The supernatants were acidified with 6 M HCl acid to pH 1–1.5 to re-precipitate and kept for 24 h. The acid-insoluble portions were settled and the precipitate was further centrifuged and washed with dilute HCl and ultrapure water until the filtrate become neutral. The precipitate was recovered and freeze dried to obtain the solid products. The two final coal-derived carbon nanomaterials (CNMs) were denoted as Coal-NG-NP and Coal-NK-NP. The HNO3 acid used in the above oxidation process was recovered by filtration and the molarity was adjusted by adding additional concentrated HNO3 acid. The recycled HNO3 acid could be used for subsequent experiments.
2.3 Characterizations techniques of the raw coal samples and their products
The proximate analysis and ultimate analysis (CHNS) of the samples were carried out an a Thermogravimetric Analyzer (Leco TGA701)37–39 and Truspec CHN Macro Determinator (Leco: 630-100-300),40 respectively. The sulfur was determined by a Dual Range Sulfur Analyzer (Leco S-144DR: accuracy ±0.02)41 and the oxygen contents was calculated by difference. For scanning electron microscopic (SEM) analysis, the samples were examined in a scanning electron microscope (Model no. JSM-6390LV; JEOL). TEM images were taken using High Resolution-Transmittance Electron Microscope (HR-TEM; Joel JEM-2100, resolution: 1.9 Å to 1.4 Å, accelerating voltage: 60–200 kV in 50 V steps). The SEM and HR-TEM micrographs were further developed by using the “Image J” program (software version 1.47).42 The X-ray diffraction spectra were obtained in an X-ray powder diffractometer (type: JDX-11P3A, JEOL: Japan) with the start angle 2°, stop angle 75°, and step angle 0.05° with a scanning rate of 1° per minute and target was Co (λ = 1.7902 Å). The Raman analysis was performed on a laser micro-Raman system (Make: Horiba Jobin Vyon, Model LabRam HR). Fourier transform infrared (FT-IR) spectra were recorded in transmittance mode with 4 cm−1 spectral resolution using a FT-IR spectrophotometer (IR Affinity-1, Shimadzu, Japan) and IR solution software. XPS analysis of the samples was carried out in an ‘ESCALAB 250 spectrometer’ (Thermo-VG Scientific) using a monochromatic Al Kα X-ray source (1486.6 eV) and typical energy resolution of 0.4–0.5 eV at full width at half maximum (FWHM). Solid-State 13C NMR of the samples was obtained from an ECX400-JEOL 400 MHz high resolution multinuclear FT-NMR spectrometer. The non-isothermal analyses (TGA-DTA) of the samples were carried out in a thermal analyzer (Model: Netzsch STA 449F3) in an air atmosphere at a heating rate of 5 °C min−1 from room temperature to 1000 °C using an Al2O3 crucible. Ultraviolet-visible spectroscopy (UV-Vis) of the samples in ultrapure water was carried out in an UV-visible spectrophotometer (Analytikjena, SPECORD-200, Germany). The fluorescence (FL) spectra of the samples in ultrapure water solution were recorded in an F-2700 FL spectrophotometer (2423-008).2.4 Photocatalytic degradation experiments
In this study, 2-nitrophenol (2-NP) was selected as a model organic pollutant to evaluate the photocatalytic efficiency of the coal-derived CNMs (i.e. Coal-NG-NP and Coal-NK-NP) under natural sunlight irradiation. The photocatalytic degradation experiment was performed in a broad pH range and different concentrations with an initial concentration of 2-NP of 0.1 mM with value subsequently changed to 0.2, 0.3, and 0.4 mM. All the experiments were carried out on bright sunny days, between 10:00 and 14:00 hours in Jorhat city, Assam, Northeastern region of India. Both the CNMs were dispersed in ultrapure water (5 g L−1) and mixed in a vortex mixture. For the typical photocatalytic reaction, 30 mL of aqueous suspension of 2-NP and CNMs mixture was stirred in a 50 mL round bottom flask in the dark for 120 min to ensure the adsorption/desorption equilibrium and then the reaction mixture was stirred under sunlight irradiation for 120 min. During the experiment, 2 mL of the reaction mixture was collected from each reaction mixture at different time intervals and the supernatant of each sample was separated using a membrane filter. The residual concentration of the 2-NP in every solution fraction was determined in the UV-visible spectrophotometer by measuring the absorbance at a wavelength of 283 nm. The CNMs were also collected from the reaction mixture by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to determine the sustainability of the CNMs for several times. The photocatalytic degradation efficiency of both these CNMs was evaluated by the following equation.
000 rpm to determine the sustainability of the CNMs for several times. The photocatalytic degradation efficiency of both these CNMs was evaluated by the following equation.| Degradation efficiency (%) = [1 − Ct/Co] × 100 | (1) |
3. Results and discussion
3.1 Physico-chemical characterizations
The physico-chemical characteristics of the raw coal samples (i.e. Coal-NG and Coal-NK) and CNMs (i.e. Coal-NG-NP and Coal-NK-NP) are summarized in Table 1. Both the raw coal samples contain significant amounts of carbon and oxygen along with small amounts of hydrogen and nitrogen, which indicate the coals to be of subbituminous type.29,43 It is observed that the ash yields and sulfur contents considerably decreased after the oxidation and extraction processes, which is similar to a previous study.29 Fig. 1 illustrates a schematic diagram adopted for the preparation of these CNMs from the low-quality Indian coals.| Samples | Proximate analysis (%) | Ultimate analysis (%) | TS (%) | Final product yields (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As received | Dry ash free basis (d.a.f.) | ||||||||||||
| M | Ash | VM | FC | C | H | N | C | H | N | O | |||
| Coal-NK | 4.20 | 18.67 | 30.68 | 46.45 | 61.20 | 4.51 | 1.30 | 79.35 | 5.85 | 1.69 | 31.45 | 3.26 | — |
| Coal-NK-NP | 17.12 | 2.63 | 43.00 | 37.25 | 51.80 | 5.54 | 0.08 | 64.55 | 6.90 | 0.10 | 43.96 | 1.51 | 76.25% |
| Coal-NG | 9.29 | 3.80 | 41.69 | 45.23 | 68.80 | 6.12 | 1.10 | 79.16 | 7.04 | 1.27 | 32.74 | 3.59 | — |
| Coal-NG-NP | 12.46 | 1.23 | 46.25 | 40.06 | 55.50 | 5.64 | 0.70 | 64.30 | 6.53 | 0.81 | 44.54 | 1.50 | 82.00% |
3.2 Observations from electron beam analysis (SEMEDX and TEM/HRTEM)
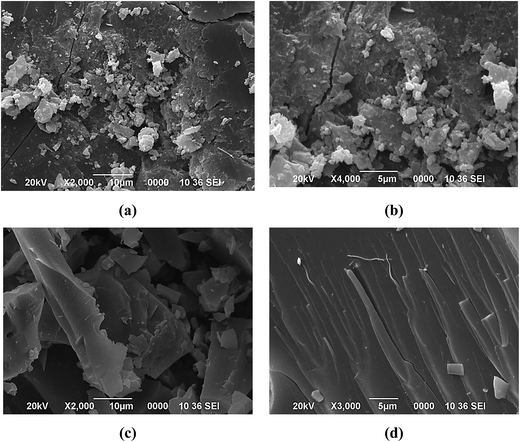
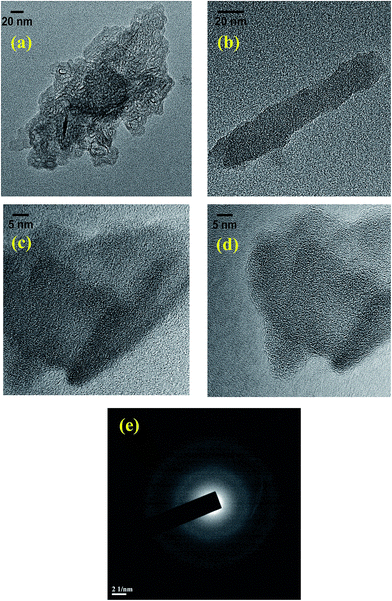
The morphology of the raw coal samples and CNMs was characterized by SEM and TEM techniques. The SEM images of the raw coal samples show the irregular size and shape distribution of the particles ranging from 1 to 50 of micron in diameter (see Fig. S-1 in ESI†) and after the oxidation-cum-extraction process, the surface of these particles appears to be smooth, wrinkled and showing a sheet-like structure (Fig. 2a–d), which is similar to that of graphite-like flakes.26 The elemental composition was also characterized by SEM-EDX (see Fig. S-1 and Table S-1 in ESI†), and the results revealed that the weights (%) of oxygen and sodium increased after the treatment, which may be due to the oxidation followed by alkaline extraction of the raw coals in the presence of HNO3 and NaOH, respectively. From the EDX study, it is observed that the CNMs (i.e. Coal-NG-NP and Coal-NK-NP) mainly consist of carbon and oxygen, indicating the formation of graphite-like carbonaceous material in this process.The morphologies of the Coal-NG-NP sample, as observed in the HRTEM images clearly show the cluster of onion-like fullerene structures (Fig. 3a–f).20,44,45 The onion-like fullerene structure displays quasi-spherical, polyhedral type morphologies with a hollow center (Fig. 3a–f). The size of the onion-like fullerene is estimated to be in the range of about 5–20 nm. The outer diameter of these onion-like structures is estimated to be in the range of 3–15 nm.
The HRTEM images of the onion-like fullerene structures also display the degree of graphitization and the graphite sheets present are wavy in nature, which act as a building block during the growth (Fig. 3a–f). The images show that most of the spherical rings are completely closed, but not in all places. Most of the onion-like rings are observed to be lying over another, but some of the rings are fused with another onion-like ring (Fig. 3a–e), which may be due the fact that a rapidly growing carbon net is likely to close imperfectly and begin a second shell before the first is completed.20
Fig. 4a and b show the mean profile interlayer distance of 0.363 nm, which is close to that of graphite (0.336 nm).20 The interlayer distance for the onion-like particles is not constant, and in some places the interlayer distance is wider, which may be due to the incorporation of sp3-carbon and sp2-carbon.46 Some spiral shapes are also observed on the surface of the onion-like fullerene structure, where the growth of new shells may take place (Fig. 4b). However, the corresponding selected area electron diffraction (SAED) pattern also reveals the existence of amorphous carbon along with the onion-like fullerene in the products (Fig. 4c).
 | ||
| Fig. 4 Profile of the variation of the intensity levels over the spiral structure (a) of the onion-like fullerene (HRTEM image; (b)) and the corresponding SAED pattern (c) of Coal-NG-NP. | ||
The onion-like fullerene structure observed in the TEM images seems to be stable and remain unchanged from the tilt experiment in the TEM. Thus, it is not reasonable to suggest that the onion-like structure is formed by rearrangement of amorphous carbon under the normal electron beam conducted in the HRTEM experiment (accelerating voltage: 60–200 kV in 50 V steps) and any organic materials present in the Coal-NG-NP. The organic material may not be stable under the electron beam of the TEM and re-arrangement of amorphous carbon to spherical shape may take place only under a strong electron beam irradiation (300 kV, high current density).44,45 The onion-like fullerene structure obtained is similar to the onion-like fullerene synthesized by means of a plasma method.20
Fig. 5a–d show that the Coal-NK-NP sample contains clusters of chemically converted graphene-like nanosheets.28,47 However, the onion-like carbon shape is not observed. HRTEM images show that the graphene-like nanosheets may be a single layer graphene and have no obvious lattice structure (Fig. 5c and d). The non-crystalline structure may be due to the high oxygen content, which is also observed in the FTIR and XPS studies. The corresponding selected area electron diffraction (SAED) pattern reveals the existence of some amorphous carbon incorporated within the graphene layer (Fig. 5e). These TEM images of the graphene-like nanosheets are similar to those of the TEM image of graphene sheets reported in literature.28,47,48
From the electron beam studies, it is observed that the present process of oxidation, followed by alkaline extractions (OCE process) may facilitate the formations of onion-like fullerenes and chemically converted graphene-like nanosheets from the low-quality coals used in this experiment. The novelty of these products is their simple preparation technique from the low-quality subbituminous coals.
To further confirm the formation of onion-like fullerenes and chemically converted graphene-like nanosheets from the coals, these CNMs were further characterized by using XRD, Raman, FTIR, XPS, solid-state 13C NMR, UV-visible spectroscopy, and FL spectroscopy.
3.3 X-ray diffraction
The raw coal (see Fig. S-2 in ESI†) and CNMs (Fig. 6a) were characterized by X-ray diffraction (XRD) techniques for qualitative assessment of the minerals and graphitic phase. The assessment of the d-values obtained from the XRD spectra was carried out with the help of the literature available.49,50 The major minerals present in the raw coal samples were found to be hematite (H), quartz (Q), muscovite (M), and kaolinite (K) (see Table S-2 in ESI†). | ||
| Fig. 6 (a) XRD pattern of the Coal-NG-NP (upper) and Coal-NK-NP (lower); (b) Raman spectra of Coal-NK (red), Coal-NG (blue), Coal-NG-NP (green) and Coal-NK-NP (black). | ||
However, after the OCE process, the XRD patterns of the samples (CNMs) are considerably changed and show a broad peak centered at around 26.7°, which is close to the peak observed for graphite at 26.4°,20 and slightly shifted away from that of a possible graphite peak and may be due to the presence of some graphite-like structure embedded within the amorphous carbon matrix and the introduction of abundant oxygen containing functional groups (mainly carboxyl and hydroxyl group).4,27,51 The d-spacing value of the Coal-NG-NP and Coal-NK-NP are found to be 3.78 Å and 3.83 Å, respectively, which is a little higher than the d-spacing value of graphite (3.36 Å).20 It might also be due to the presence of abundant oxygen containing functional groups and some structural curvature or structural defect in the CNMs.20 The XRD patterns of the coal sample after the OCE process are found to be quite similar with the XRD patterns of the reported coal-derived carbon nanomaterials.4,27–29,47,51
3.4 Raman spectroscopy
Raman spectroscopy is a very effective and high output technique for the identification of various forms of carbon including fullerene and graphene-like carbon.52,53 Fig. 6b shows the Raman spectra of the coal samples before and after the OCE process. The Raman spectra of the raw coal samples show D and G peaks, and the integrated intensity ratio of amorphous D-bands to crystalline G-bands (ID/IG) for the Coal-NG and Coal-NK is estimated to be about 1.83 and 2.00, respectively (see Fig. S-3 and Table S-3 in ESI†). It is therefore concluded that Coal-NG contains more graphitic-like stacking domains compared to that of Coal-NK. The 2D and 2G peaks are not observed in the raw coal samples, which also reveals that the coal samples are of sub-bituminous rank and have a higher proportion of aliphatic carbon and smaller polyaromatic domains.27The Raman spectra of the coal samples after the OCE process shows mainly two characteristic bands appearing at 1596 cm−1 (G-band) and 1384 cm−1 (D-band) for Coal-NG-NP; and 1595 cm−1 (G-band) and 1382 cm−1 (D-band) for Coal-NK-NP. The G-band originated from the vibration of the sp2-hybridized carbon framework in the 2D hexagonal lattice of graphite cluster and D-band originated from a lattice defect including the sp3 hybridized carbon.53 The G-bands correspond to the first-order scattering of the E2g stretching mode of graphite. The D-band is due to the residual ill-organized graphite.29 The relative integrated intensity ratio of the D and G-bands (ID/IG) are found to have increased to 2.79 for Coal-NK-NP, which may be due to the introduction of the defects to the basal planes and edges caused by the use of a strong acid (i.e. HNO3) during oxidation.27,54–56 The ID/IG ratio of Coal-NG-NP sample is decreased to 1.81 due to the increase in its graphitization. The higher value of the ID/IG ratio indicates the smaller crystalline size and the low graphitization degree that resulted from the large number of defects and edges in the samples and this is in good agreement with the HRTEM and XRD results. It is observed that the Coal-NK-NP sample has a higher defect density compared to that of Coal-NG-NP. The Raman spectrum of the prepared CNMs are found to be quite similar with those for carbon-based onion-like fullerene,52,54,55,57–59 graphene-like carbon,26,56 and other coal-derived carbon nanomaterials.4,27–29,47,51
3.5 FT-IR spectroscopy
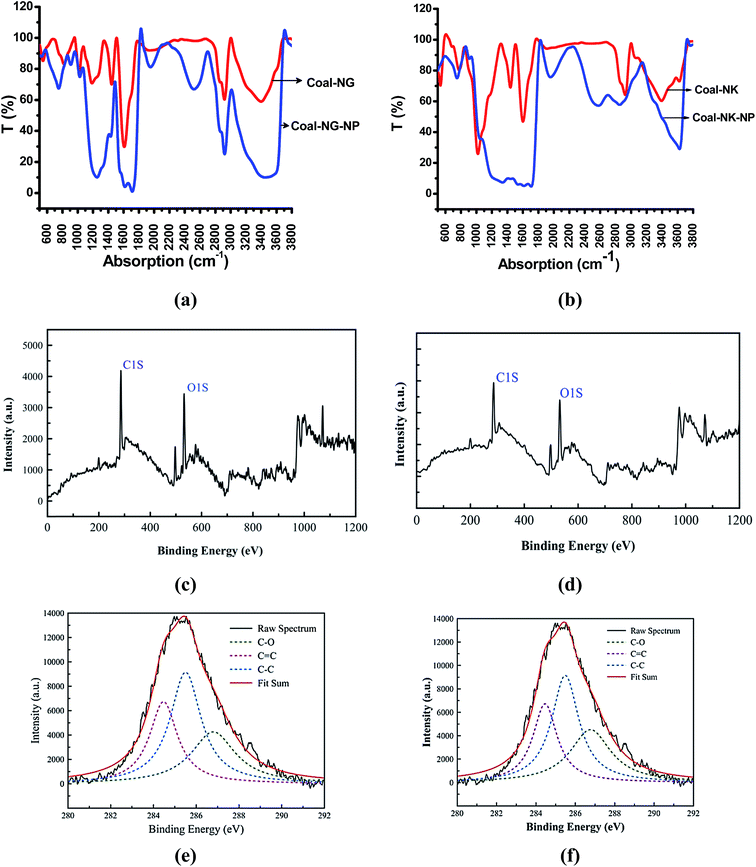
FT-IR spectroscopy was performed to investigate the surface functional groups and type of chemical bonding environment of the raw coal samples and CNMs. The FTIR spectra of the raw coal samples show the presence of obvious absorption peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O and O–H groups (Fig. 7a and b) (see Table S-4 in ESI†). The coal-derived carbon nanomaterials usually show a distinct FT-IR absorptions depending upon its geometry29. Some of the drastic changes are observed in the FT-IR spectra for the CNMs obtained by this OCE process (Fig. 7a and b). The broad absorption peaks are observed at around 3463 cm−1 (Coal-NG-NP) and 3639 cm−1 (Coal-NK-NP), which is mainly due to the O–H and N–H groups.60 The peaks at around 2923, 2851 cm−1 (Coal-NG-NP), and 2912, 2845 (Coal-NK-NP) are due to the CH2 asymmetric vibration and CH3 symmetric vibration bonded to aromatic group, respectively.61 The broad peak at around 2523 cm−1 (Coal-NG-NP) and 2541 cm−1 (Coal-NK-NP) is due to the intramolecular O–H stretching in the CNMs.
C, C–O and O–H groups (Fig. 7a and b) (see Table S-4 in ESI†). The coal-derived carbon nanomaterials usually show a distinct FT-IR absorptions depending upon its geometry29. Some of the drastic changes are observed in the FT-IR spectra for the CNMs obtained by this OCE process (Fig. 7a and b). The broad absorption peaks are observed at around 3463 cm−1 (Coal-NG-NP) and 3639 cm−1 (Coal-NK-NP), which is mainly due to the O–H and N–H groups.60 The peaks at around 2923, 2851 cm−1 (Coal-NG-NP), and 2912, 2845 (Coal-NK-NP) are due to the CH2 asymmetric vibration and CH3 symmetric vibration bonded to aromatic group, respectively.61 The broad peak at around 2523 cm−1 (Coal-NG-NP) and 2541 cm−1 (Coal-NK-NP) is due to the intramolecular O–H stretching in the CNMs.
The sharp absorption peaks at around 1720 and 1600 cm−1 of the CNMs are mainly due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and aromatic C
O group and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations (G-band of graphite).61 The peak intensity at around 1600 cm−1 of the coal samples is increased after the OCE process, and also broadened due to the formation of C
C stretching vibrations (G-band of graphite).61 The peak intensity at around 1600 cm−1 of the coal samples is increased after the OCE process, and also broadened due to the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds during the process. A broad peak observed at around 1440 cm−1 is mainly due to the COO− and CH3 groups. A broad and intense peak observed at around 1250 cm−1 is due to C
C double bonds during the process. A broad peak observed at around 1440 cm−1 is mainly due to the COO− and CH3 groups. A broad and intense peak observed at around 1250 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of COOH and the presence of carbon nanomaterials.29 The peaks at around 1034–1042 cm−1 are mainly due to the C–O stretching and amorphous carbon or mineral matters in the samples.29
O stretching of COOH and the presence of carbon nanomaterials.29 The peaks at around 1034–1042 cm−1 are mainly due to the C–O stretching and amorphous carbon or mineral matters in the samples.29
Thus, the FT-IR analysis reveals that the CNMs contain abundant oxygen-containing functional groups (i.e. carboxyl, carbonyl and hydroxyl group) and sp2 aromatic structure units, which is also in agreement with the SEM-EDX data. Furthermore, the relative peak intensity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and C
O group and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group particularly become strong after the OCE process. The FT-IR data of the CNMs are observed to be similar with those of onion-like fullerene structure,62,63 graphene nanosheets, and other coal-derived carbon nanomaterials.4,27,29,47,51
C group particularly become strong after the OCE process. The FT-IR data of the CNMs are observed to be similar with those of onion-like fullerene structure,62,63 graphene nanosheets, and other coal-derived carbon nanomaterials.4,27,29,47,51
3.6 X-ray photoelectron spectroscopy (XPS)
The survey of X-ray photoelectron (XPS) spectra of the C1s peak of the coal samples before the OCE process shows the presence of only C–O containing groups (see Fig. S-4a and b, in ESI†). The XPS analyses of the coal samples after the OCE process are also shown in Fig. 7c–f. The analyses indicate that the coal-derived CNMs consist of carbon (C1s peak at 285 eV) and oxygen (O1s peak at 533 eV) (Fig. 7c and d). The high resolution C1s XPS deconvoluted spectra of the CNMs shows that the C1s XPS peak splits into three main peaks, which are located at 284.41 (C–C sp2), 285.54 (C–C sp3), and 286.98 (C–O) for Coal-NG-NP (Fig. 7e), and 284.46 (C–C sp2), 285.49 (C–C sp3), and 286.77 (C–O) for Coal-NK-NP (Fig. 7f), respectively. The binding energies of sp2 and sp3 components of the coal samples after the OCE process for the C1s peak are found to be in good agreement with the binding energies of 284.4 and 285.2 eV detected for C1s peak of pure graphite and diamond51 and is also in accordance with the XPS results of the coal-derived carbon nanomaterials reported in literature.1,4,27 However, the sp3 component can also be contributed to the amorphous carbon present, which is also observed in the SAED pattern of the TEM analysis. The percentages (%) of sp2-C, sp3-C, and sp3/sp2 ratio are estimated to be about 57.10%, 42.90%, and 0.75% respectively for Coal-NG-NP; and for Coal-NK-NP these are estimated to be about 58.19% (sp2-C), 41.80 (sp3-C), and 0.71 (sp3/sp2). The high resolution O1s XPS fitting spectra show the presence of carboxyl (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) and C–O, which are located at 532.24 eV and 531.90 eV for Coal-NG-NP and Coal-NK-NP, respectively (see Fig. S-4c and d in ESI†). The functionalization of the coal samples after the OCE process was also confirmed by the increase in the intensities of C–O, C
C–O) and C–O, which are located at 532.24 eV and 531.90 eV for Coal-NG-NP and Coal-NK-NP, respectively (see Fig. S-4c and d in ESI†). The functionalization of the coal samples after the OCE process was also confirmed by the increase in the intensities of C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H vibrations modes in the FTIR spectrum.
O and O–H vibrations modes in the FTIR spectrum.
3.7 13C cross polarization-magic angle spinning (CP-MAS) NMR spectroscopy
The solid-state 13C NMR CP-MAS spectra of the raw and CNMs were recorded in order to obtain the least qualitative information about the nature of the carbon atom (see Fig. S-5a–d in ESI†). The NMR analysis shows that the major portions of carbon in the CNMs are in an aromatic structure. The raw coal samples, after the OCE process (i.e. Coal-NG-NK and Coal-NK-NP), show the prominent band with a maximum peak at ∼30 ppm for aliphatic carbon64 and a signal at ∼128 ppm for the un-substituted aromatic carbon (sp2 carbon) along with a small peak at ∼170 ppm, which is mainly due to the formation of the carbonyl group in aromatic carbon.28,64 The new peak formed in the range of 230–250 ppm after the oxidation-cum-extraction process (see Fig. S-5c and d in ESI†) is due to the formation of aromatic structural units during the process.643.8 Thermal analysis (TGA-DTA)
Fig. 8a and b represent the non-isothermal thermogravimetric analysis-differential thermal analysis (TGA-DTA) of CNMs (i.e. Coal-NG-NP and Coal-NK-NP). It is notable that the majority of the mass loss occurs within the temperature range of 500–580 °C. In the TGA-DTG curve, the weight loss observed at a temperature of 100 °C is due to the release of water from the samples. At the temperature range of 200–400 °C, slow weight loss is caused due to the loss of a small amount of amorphous carbon during the non-isothermal studies. Distinct exothermic peaks were observed when temperatures were above 400 °C in the Coal-NG-NP (one big and one small peaks) (Fig. 8a) and Coal-NK-NP (two big and one small peaks) (Fig. 8b) in which the maximum weight loss occurred due to the burning of aromatic as well as amorphous carbons and it is calculated to be about 86.44% and 78.90%, respectively. The ignition temperature of the CNMs (Coal-NG-NP and Coal-NK-NP) is found to be approximately in the range of 400–500 °C, which is quite similar with the burning temperatures of fullerene (425 °C),25 graphene oxide,65 and graphene nano-sheets.65 However, the thermal stability of the Coal-NG-NP and Coal-NK-NP is found to be less in comparison to that of graphite powder, which may be due to the presence of amorphous carbon, introduction of abundant oxygen-containing functional group, and some structural curvature or structural defect in the coal-derived carbon nanomaterials (CNMs).25,65 | ||
| Fig. 8 TGA-DTA characterizations of coal-derived CNMs (a) TGA (black), DTG (red) and DTA (blue) of Coal-NG-NP; (b) TGA (black), DTG (red) and DTA (blue) of Coal-NK-NP. | ||
3.9 UV-visible and fluorescence (FL) spectroscopy
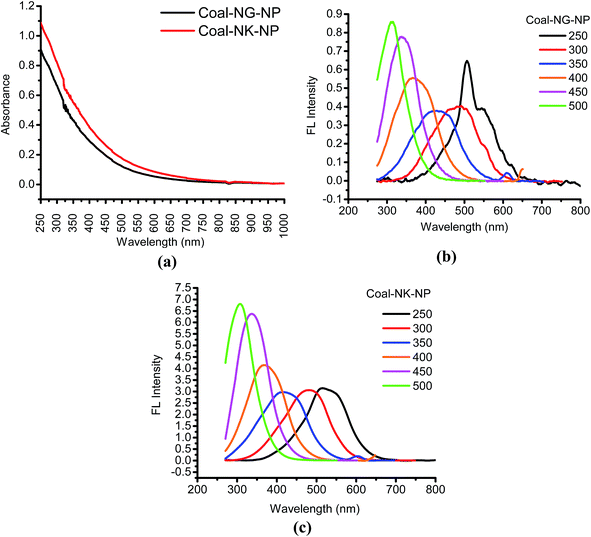
The photo-physical characterizations of the CNMs were carried out by using UV-visible and FL spectroscopic techniques. Fig. 9a shows the UV-visible absorption spectra of the coal samples after the OCE process. The bands appear at around 250–350 nm and are due to the excitation of pi-electrons (π → π*) of the aromatic π system, while a shoulder at 300 nm attributes to the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds or other connected groups. The broad absorption with a gradual change up to a long wavelength indicates the existence of band tails by defect states.27
O bonds or other connected groups. The broad absorption with a gradual change up to a long wavelength indicates the existence of band tails by defect states.27
Fig. 9b and c show the FL spectra of the CNMs. Both the FL properties of Coal-NG-NP (Fig. 9b) and Coal-NK-NP (Fig. 9c) are found to be excitation dependent. In both the samples, the FL peaks show a significant blue-shift with the gradual increase of excitation wavelength from 250 to 500 nm. The shifting of the peaks is caused by to non-uniform size of the CNMs.27 The FL behavior of both the CNMs are found to be almost similar, however, the FL peak intensity of the Coal-NK-NP is higher than the Coal-NG-NP, which may be due the high Raman ID/IG value of Coal-NK-NP.27 The wide FL emission spectrum is also observed for both the CNMs, which may be due to the presence of different sizes of sp2-domains in the samples.66 The origin of the FL properties of the samples may arise from the conjugated sp2 π-domains, and surface/edge state.27,66 However, further work on the quantum confinement effect or conjugated π-domains, surface state, molecule state, and cross linking enhanced emission (CEE) effect on FL behavior are warranted. The FL properties of the CNMs are quite similar to those reported for carbon-based nanomaterials synthesized from coals by using chemical methods4,27,28,51 and expected to show excellent photocatalytic activity.
The UV-visible diffuse reflectance (UV-vis DRS) spectra of the synthesized coal-derived CNMs were also examined and it was found that they show the characteristic behavior of semiconductor-type materials (see Fig. S-6 in ESI†).
All of the characterization results reveal that the oxidation of low-quality Northeast Indian coals in HNO3, followed by extraction in NaOH, produces CNMs (Coal-NG-NP and Coal-NK-NP) consisting of onion-like fullerenes and chemically converted graphene-like nanosheets incorporated with some amorphous carbon of predominantly sp2-carbon. All the characterization results observed are quite similar with those reported for carbon nanomaterials.4,27,28,51 However, the structural differences are observed between the CNMs, which may be due to the differences in the physico-chemical properties of the raw coal. The relative intensity of the “disordered” D-band to the “crystalline” G-band (ID/IG) for the Coal-NG is calculated to be about 1.83, which is higher than that of Coal-NK (2.00), which indicates that the Coal-NG contains more graphite-like structures compared to that of Coal-NK. Also, the structural defects in Coal-NK-NP (ID/IG = 2.79) are more than was observed for the Coal-NG-NP (ID/IG = 1.83). The yield of the CNMs is calculated to be about 76.25–82.00% (wt) (Table 1) and close to the reported literature (see Table S-5 in ESI†).4,5,27,28,51
3.10 Proposed mechanism of the formation of onion-like fullerene and graphene-like nanosheets from coals
The mechanism of formation of the CNMs during the OCE process can be hypothesized and in order to describe the formation of these CNMs, the aspects of chemical structure of the coals are mandatory to take into account.The low-quality Northeast Indian coals were reported to have graphite-like polyaromatic structures.67–69 The graphitic-like structure present in these coals can be broken and separated by means of chemical treatments, such as chemical oxidation.4 The irregular and polyaromatic hydrocarbons in coal are joined by weak links and were preferentially oxidized during refluxing with HNO3 acid in this OCE process, leading to the formation of partially ordered and defective carbon layers.4 The Na+ species formed during the second step of the process (extraction) intercalated between the ordered and disordered layers in the coal structure and rearrangement of the layers may also occur.29 The sodium alumino-silicate (including other mineral matters) formed during NaOH treatment was removed during the HCl treatment.29 In this way a large amount of aromatic fragments, including C1 or C2 carbon units from coal may be released and rearrangement and aggregation of these fragments formed the onion-like fullerenes and chemically converted graphene-like nanosheets during the OCE process (see Fig. S-7 in ESI†). The aromatic fragments may be nucleated at the surface of the mineral matter or catalytic species, which are generally present in coals such as FeS2, Co, Ni, Cu etc. via a kind of synergic effect.9 The smaller aromatic fragments (C1 and C2) carbon units act as building blocks in the innermost shell and continue to bond with the nucleation and finally give the onion like-structure.20 Moreover, the high sulfur contents in the Northeast Indian coal samples could be helpful for the formation of active sites on the metal catalyst in the forming metal-sulfur eutectic, which results in growth of the coal-derived carbon nanomaterials.10 The presence of amorphous carbon in the onion-like fullerene produced in the OCE process suggest that the graphitization trend occurs around the onion-like fullerene curve and grows from the center to the surface.20
3.11 Photocatalytic degradation activity of the CNMs
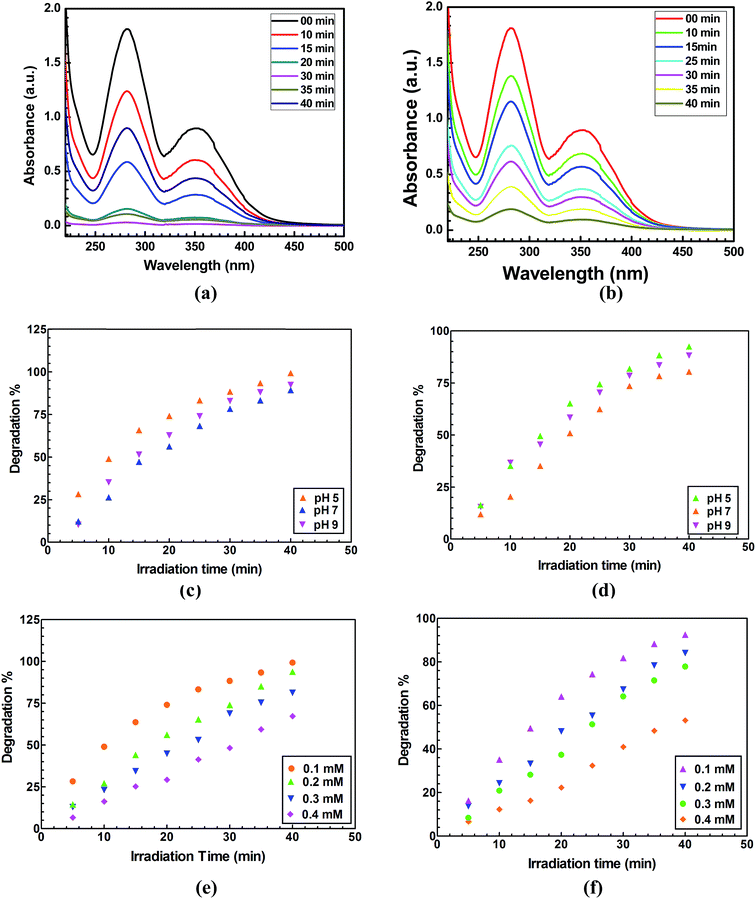
The photocatalytic activity of the CNMs (Coal-NG-NP and Coal-NK-NP) were evaluated by degradation of 2-NP in aqueous conditions under a direct natural sunlight irradiation medium. The UV-visible spectral changes for the photocatalytic degradation of 2-NP in he presence of Coal-NG-NP and Coal-NK-NP nanomaterials and are shown in the Fig. 10a and b. It is clearly seen that Coal-NG-NP completely degrades 2-NP within a very short time period (40 min) under natural sunlight. A degradation of more than 90% was achieved within 40 min using Coal-NK-NP as photocatalyst. The degradation of 2-NP in the presence of Coal-NG-NP and Coal-NK-NP nanomaterials were studied considering the different parameters like what the effect is of varying the initial pH, initial 2-NP concentration and catalyst loading. The kinetics of the degradation process and reusability of the catalyst was also investigated. The adsorption of 2-NP on the Coal-NG-NP and Coal-NK-NP in the dark, to understand the adsorption potential of these coal-derived CNMs, was also investigated. The adsorption efficiency was found to be 26.54% and 21.43% for Coal-NG-NP and Coal-NK-NP, respectively at 40 min degradation time (Fig. S-8a, ESI†). However, the 2-NP degradation efficiency was observed to be 99.34% and 92.49% within the 40 min time period when Coal-NG-NP and Coal-NK-NP were used as a photocatalyst under sunlight irradiation (details description is provided in the ESI and Fig. S-8a and b†).The effect of pH of the solution on the degradation also plays an important role in the photocatalytic degradation of 2-NP. The effect of solution pH in the degradation of 2-NP was investigated by varying the initial pH of the solution at fixed 2-NP concentration (0.1 mM) and catalyst loading of 0.5 g L−1 (Fig. 10c and d). The degradation of 2-NP was achieved and found to be 99.34% using Coal-NG-NP at pH 5 followed by 92.43% at pH 9. However, a lower photodegradation efficiency was observed at pH 7 (89.32%). Similarly for Coal-NK-NP nanomaterial, the highest degradation was achieved at acidic pH (92.49% of degradation at pH 5) and then it decreased at pH 7 (80.44%), and then slightly increased at pH 9 (88.23%). The difference in the degradation efficiencies of these CNMs may be due to the difference in concentration of the hydrogen ions and hydroxyl ions at different pH values.
The photodegradation of 2-NP by the Coal-NG-NP and Coal-NK-NP was evaluated by varying the initial concentration of 2-NP in the range 0.1 mM to 0.4 mM at a fixed pH value of 5 and catalyst loading of 0.5 g L−1. The photodegradation efficiency decreased gradually with an increase of 2-NP concentration from 0.1 mM to 0.4 mM (Fig. 10e and f). 99.34% of degradation efficiency was observed with Coal-NG-NP at 0.1 mM concentration of 2-NP, whereas 67.23% of the degradation efficiency was obtained with 0.4 mM concentration of 2-NP (Fig. 10e). 92.49% of degradation efficiency was observed when Coal-NK-NP was used as a photo-catalyst, which gradually decreased to 53.21% at pH 9 (Fig. 10f). Thus, the photodegradation efficiency of the CNMs decreases with the increase the concentration of 2-NP due to the availability of surface active sites.32
The effect of loading Coal-NG-NP and Coal-NK-NP on the degradation of 2-NP was investigated at a fixed concentration of 2-NP at pH 5 under sunlight irradiation and also by varying the amount of catalyst from 0.1 g L−1 to 1 g L−1 (Fig. 11a). The 2-NP degradation efficiency gradually increased from 0.1 g L−1 to 0.5 g L−1 of catalyst loading and then remained almost the same up to 1 g L−1. The agglomeration of catalyst at a higher concentration results in a decrease of available reactive sites, therefore optimum catalyst loading was achieved with 0.5 g L−1 for Coal-NK-NP and Coal-NG-NP nanomaterials.
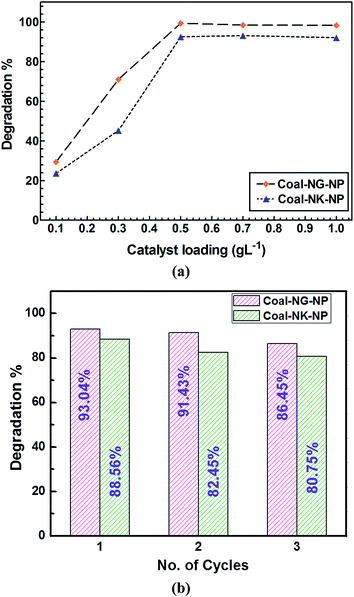 | ||
| Fig. 11 (a) Effect of loading of Coal-NG-NP and Coal-NK-NP on photodegradation of 2-NP; (b) reusability study of Coal-NG-NP and Coal-NK-NP for degradation of 2-NP. | ||
3.12 Investigation of intermediate and final products
The initial and the final products in the 2-NP degradation (concentration 0.1 mM, catalyst loading 0.5 g L−1 at pH 5) were analyzed by using ion chromatography (Metrohm Compact IC Pro 882, Switzerland) (see Fig. S-9 in ESI†). Anion chromatography analysis was carried out for 2-NP molecules before and after degradation under sunlight irradiation. It was observed that the peak corresponding to a chloride (Cl−) anion, where the Cl− ion existed as a counter ion in aqueous dye solution, appears in the samples that were not subjected to sunlight irradiation. However, the peaks corresponding to the nitrate (NO3−) anions were observed in the degraded samples of the 2-NP. Therefore, it could be inferred that the C–N bonds in the 2-NP molecules dissociates and nitrogen atoms get oxidized and directly converted into inorganic harmless NO3− anions and other inorganic molecules like CO2 and H2O.3.13 Degradation kinetics
The kinetics of degradation of 2-NP by using Coal-NG-NP and Coal-NK-NP nanomaterial are shown in the Fig. S-10, ESI.† The kinetics of 2-NP degradation was investigated by varying the initial pH of the solution. The Langmuir–Hinshelwood pseudo first order kinetic model is fitted for 2-NP degradation process as follows.| ln(Co/C) = kt | (2) |
| Catalyst | pH | k (min−1) | Degradation (%) | R2 |
|---|---|---|---|---|
| Coal-NG-NP | 5 | 0.0776 | 99.34 | 0.9921 |
| 7 | 0.0559 | 89.32 | 0.9892 | |
| 9 | 0.0645 | 92.43 | 0.9933 | |
| Coal-NK-NP | 5 | 0.0647 | 92.49 | 0.9909 |
| 7 | 0.0408 | 80.44 | 0.9843 | |
| 9 | 0.0535 | 88.23 | 0.9934 |
3.14 Reusability of the photocatalyst
Recycling of the photocatalyst (i.e. Coal-NK-NP and Coal-NG-NP) after photocatalytic degradation is an important parameter to evaluate the efficiency of the catalyst for long term use. The recyclability test was performed for Coal-NK-NP and Coal-NG-NP nanomaterials over 2-NP degradation at catalyst loading of 0.5 g L−1 and concentration of 2-NP of 0.1 mM at pH 5 (Fig. 11b). After the 1st run of photocatalytic treatment, the reaction mixture was filtered and the catalyst part was washed with ultrapure water followed by acetone and then dried in an air oven at 70 °C. The recovered catalyst was mixed with fresh 2-NP solution and a similar degradation process was monitored for 3-cycles under sunlight irradiation. A significant loss of photocatalytic activity was found in every cycle for both Coal-NG-NP and Coal-NK-NP nanomaterials due to the dissolution of small particles in the aqueous solution in the respective cycles. The 2-NP degradation efficiency was 93.04, 91.43, and 86.45% for 1st, 2nd, and 3rd cycles, respectively for Coal-NG-NP. For Coal-NK-NP nanomaterials, it was found to be 88.56, 82.45, and 80.75%, respectively.Despite the variety of carbon materials developed for the waste water treatment, their practical application is limited due to the high production cost of the material as well as the less effective and economical technique used.30,31 In addition to that, the adsorption and coagulation technology does not completely destroy the pollutants to less toxic organic, as well as inorganic compounds.30 The present report on the photodegradation of 2-NP (up to 93%) in a water system under natural sunlight, by using the synthesized coal-derived CNMs is higher than that of the degradation of p-nitrophenol (57%) in the presence of biochars,70 adsorption of p-nitrophenol on activated carbon (92%),71 degradation of p-nitrophenol (90%) from aqueous solution using granular activated carbon under microwave,72 and also close to the photocatalytic degradation of 4-nitrophenol (100%) using titanium dioxide (TiO2) composite nanowires, encapsulated with graphene and palladium nanoparticles.73 At this point, the use of the prepared coal-derived CNMs (onion like fullerene and chemically converted graphene like nanosheets) for the wastewater treatment may be a futuristic viable process in coal utilization technology.
4. Conclusions
In summary, high value materials like onion-like fullerenes and graphene-like nanosheets could be prepared from the low-quality coals by using a simple and less-expensive oxidation-cum-extraction process (OCE). The process might also be an alternative process for large-scale production of carbon nanomaterials (CNMs) from coals at a lower cost instead of the drastic and expensive methods available. These coal-derived carbon nanomaterials could also be effectively utilized in photodegradation of hazardous 2-nitrophenols (up to 93%) in a water system at different pH. However, the more detailed morphology such as the spherical shape (thickness of particle), hexagonal and pentagonal carbon rings is to be studied along with the photodegradation mechanism for better applicability of the materials.Acknowledgements
The authors are grateful to Director, CSIR-NEIST and Dr Bimala P Baruah for his constant encouragement during the research work. The financial assistance from CSIR-New Delhi is duly acknowledged (MLP-6000-WP-III). Authors express their thankfulness to Dr S. K. Biswal, Dr Ratan Borauh, Dr Dulen Saikia, SAIF-NEHU, NMR Centre-IISc, IIT-G for their instrumental support. The authors express their gratitude to the Editor, two anonymous reviewers, and Prof. Frans Waanders for the constructive comments to improve the revised manuscript.References
- M. Monthioux and V. L. Kuznetsov, Carbon, 2006, 44, 1621–1623 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- H. W. Kroto, J. R. Heath, S. C. O'Brien and R. F. Curl, Nature, 1985, 318, 162–163 CrossRef CAS.
- Y. Dong, J. Lin, Y. Chen, F. Fu, Y. Chi and G. Chen, Nanoscale, 2014, 6, 7410–7415 RSC.
- J. S. Qui, F. Zhang, Y. Zhou, H. M. Han, D. S. Hu, S. C. Tsang and P. J. F. Harris, Fuel, 2002, 81, 1509–1514 CrossRef.
- J. S. Qiu, Y. Zhou, L. N. Wang and S. C. Tsang, Carbon, 1998, 36, 465–467 CrossRef CAS.
- J. Qiu, Y. Li, Y. Wang, T. Wang, Z. Zhao, Y. Zhou, F. Li and H. Cheng, Carbon, 2003, 41, 2170–2173 CrossRef CAS.
- J. Qiu, Y. An, Z. Zhao, Y. Li and Y. Zhou, Fuel Process. Technol., 2004, 85, 913–920 CrossRef CAS.
- J. Qiu, Y. Li, Y. Wang, F. Wu, H. Cheng, G. Zheng and Y. Uchiyama, Solid Fuel Chem., 2004, 49(2), 874–875 CAS.
- J. Qiu, Z. Wang, Z. Zhao and T. Wang, Fuel, 2007, 86, 282–286 CrossRef CAS.
- C. Velasco-Santos, A. L. Martinez-Hernandez, A. Consultchi, R. Rodriguez and V. M. Castano, Chem. Phys. Lett., 2003, 373, 272–276 CrossRef CAS.
- J. Yu, J. Lucas, V. Strezov and T. Wall, Fuel, 2003, 82, 2025–2032 CrossRef CAS.
- Z. Wang, Z. Zhao and J. Qiu, Carbon, 2006, 44, 1321–1324 CrossRef CAS.
- A. Dosodia, C. Lal, B. P. Singh, R. B. Mathur and D. K. Sharma, Fullerenes, Nanotubes, Carbon Nanostruct., 2009, 17, 567–582 CrossRef CAS.
- A. Aqel, K. M. M. El-Nour, R. A. A. Ammar and A. Al-Warthan, Arabian J. Chem., 2012, 5, 1–23 CrossRef CAS.
- O. I. Zelenskii, V. M. Shmal'ko, V. G. Udovitskii and A. Y. Kropotov, Coke Chem., 2012, 55, 76–81 CrossRef.
- Y. J. Tian, Y. L. Zhang, Q. Yüa, X. Z. Wang, Z. Hua, Y. F. Zhang and K. C. Xie, Catal. Today, 2004, 89, 233–236 CrossRef CAS.
- Y. Tian, Y. Zhang, B. Wang, W. Ji, Y. Zhang and K. Xie, Carbon, 2004, 42, 2597–2601 CrossRef CAS.
- S. Awasthi, K. Awasthi, A. K. Ghosh, S. K. Srivastava and O. N. Srivastava, Fuel, 2015, 147, 35–42 CrossRef CAS.
- A. B. Du, X. G. Liu, D. F. Fu, P. D. Han and B. S. Xu, Fuel, 2007, 86, 294–298 CrossRef CAS.
- Z. Guilin, M. Yuedong, S. Xingsheng and F. Shidong, Plasma Sci. Technol., 2009, 11, 487–492 CrossRef.
- J. S. Qiua, Y. Zhoua, Z. G. Yanga, D. K. Wanga, S. C. Guoa, S. C. Tsangb and P. J. F. Harrisb, Fuel, 2000, 79, 1303–1308 CrossRef.
- R. B. Mathur, C. Lal and D. K. Sharma, Energy Sources, Part A, 2007, 29, 21–27 CrossRef CAS.
- K. Moothi, G. S. Simate, R. Falcon, S. E. Iyuke and M. Meyyappan, Langmuir, 2015, 31, 9464–9472 CrossRef CAS PubMed.
- L. S. K. Pang and M. A. Wilson, Energy Fuels, 1993, 7, 436–437 CrossRef CAS.
- Q. Zhou, Z. Zhao, Y. Zhang, B. Meng, A. Zhou and J. Qiu, Fuel, 2012, 26, 5186–5192 CrossRef CAS.
- R. Ye, C. Xiang, J. Lin, Z. Peng, K. Huang, Z. Yan, N. P. Cook, E. L. G. C. Samuel, C. Hwang, G. Ruan, G. Ceriotti, A. R. O. Raji, A. A. Martí and J. M. Tour, Nat. Commun., 2013, 4, 2943, DOI:10.1038/ncomms3943.
- R. Ye, Z. Peng, A. Metzger, J. Lin, J. A. Mann, K. Huang, C. Xiang, X. Fan, F. L. G. Samuel, L. G. Errol, L. B. Alemany, A. A. Martí and J. M. Tour, ACS Appl. Mater. Interfaces, 2015, 7, 7041–7048 CAS.
- T. Das, B. K. Saikia and B. P. Baruah, Gondwana Res., 2016, 31, 295–304 CrossRef CAS.
- C. C. Wang, J. R. Li, X. L. Lv, Y. Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 CAS.
- F. Perreault, A. F. de Faria and M. Elimelech, Chem. Soc. Rev., 2015, 5861–5896 RSC.
- A. Ghosh, M. Khurana, A. Chauhan, M. Takeo, A. K. Chakraborti and R. K. Jain, Environ. Sci. Technol., 2010, 44, 1069–1077 CrossRef CAS PubMed.
- J. S. Samdani and P. Thakur, Int. J. Appl. Sci., Eng. Technol., 2014, 3, 19–24 Search PubMed.
- L. Yang, S. Luo, Y. Li, Y. Xiao and Q. K. Q. Cai, Environ. Sci. Technol., 2010, 44, 7641–7646 CrossRef CAS PubMed.
- M. T. Amin, A. A. Alazba and U. Manzoor, Adv. Mater. Sci. Eng., 2014, 825910, DOI: org/10.1155/2014/825910.
- ASTM D2013/D2013M-12, West Conshohocken, PA, 2012, http://www.astm.org.
- ASTM D3173-11, West Conshohocken, PA, 2011, http://www.astm.org.
- ASTM D3174-12, West Conshohocken, PA, 2012, http://www.astm.org.
- ASTM D3175-11, West Conshohocken, PA, 2011, http://www.astm.org.
- ASTM D3176-15, West Conshohocken, PA, 2015, http://www.astm.org.
- ASTM D5016-08e1, West Conshohocken, PA, 2015, http://www.astm.org.
- W. Burger and J. Mark, Digital Image Processing, Springer-Verlag, London, 2008 Search PubMed.
- B. K. Saikia, A. Dutta, L. Saikia, S. Ahmed and B. P. Baruah, Fuel Process. Technol., 2014a, 123, 107–113 Search PubMed.
- D. Ugarte, Nature, 1992, 359, 707–709 CrossRef CAS PubMed.
- D. Ugarte, Europhys. Lett., 1993, 22, 45 CrossRef CAS.
- S. Wada, C. Kaito, S. Kimura, H. Ono and A. T. Tokunaga, Astron. Astrophys., 1999, 345, 259–264 CAS.
- I. K. Moon, J. Lee, R. S. Ruoff and H. Lee, Nat. Commun., 2010, 73, DOI:10.1038/ncomms1067.
- M. Khenfouch, U. Buttner, M. Baïtoul and M. Maaza, Graphene, 2014, 3, 7–13 CrossRef.
- B. K. Saikia, R. L. Goswamee, B. P. Baruah and R. K. Baruah, Coke Chem., 2009, 52, 54–59 CrossRef.
- B. K. Saikia, C. R. Ward, M. L. S. Oliveira, J. C. Hower, B. P. Baruah, M. Braga and L. F. Silva, Int. J. Coal Geol., 2014, 121, 26–34 CrossRef CAS.
- J. Xiao, P. Liu and G. W. Yang, Nanoscale, 2015, 7, 6114–6125 RSC.
- W. Xiaomin, X. Bingshe, J. Husheng, L. Xuguang and I. Hideki, J. Phys. Chem. Solids, 2006, 67, 871–874 CrossRef.
- P. K. Chu and L. Li, Mater. Chem. Phys., 2006, 96, 253–277 CrossRef CAS.
- Fu-D. Han, B. Yao and Y.-J. Bai, J. Phys. Chem., 2011, 115, 8923–8927 CAS.
- M. V. K. Azhagan, M. V. Vaishampayan and M. V. Shelke, J. Mater. Chem. A, 2014, 2, 2152–2159 CAS.
- S. H. Vijapur, D. Wang and G. G. Botte, ECS Solid State Lett., 2013, 2, 45–47 CrossRef.
- W. Xiaomin, X. Bingshe, L. Xuguang, G. Junjie and I. Hideki, Diamond Relat. Mater., 2006, 147–150 Search PubMed.
- B. S. Xu, T. B. Li, P. D. Han, X. G. Liu and I. Hideki, Mater. Lett., 2006, 60, 2042–2045 CrossRef CAS.
- E. D. Obraztsova, M. Fujii, S. Hayashi, V. L. Kuznetsov, Y. V. Butenko and A. L. Chuvilin, Carbon, 1998, 36, 821–826 CrossRef CAS.
- B. K. Saikia, R. K. Boruah, P. K. Gogoi and B. P. Baruah, Fuel Process. Technol., 2009b, 90, 196–203 Search PubMed.
- A. N. Mohan and B. Manoj, Int. J. Electrochem. Sci., 2012, 7, 9537–9549 CAS.
- J. Luszczyn, M. E. Plonska-brzezinska, A. Palkar, A. T. Dubis, A. Simionescu, D. T. Simionescu, B. Kalska-szostko, K. Winkler and L. Echegoyen, Chem.–Eur. J., 2010, 16, 4870–4880 CrossRef CAS PubMed.
- L. Zhou, C. Gao, D. Zhu, W. Xu, F. F. Chen, A. Palkar, L. Echegoyen and E. S.-W. Kong, Chem.–Eur. J., 2009, 15, 1389–1396 CrossRef CAS PubMed.
- B. K. Saikia, A. Sharma, K. Khound and B. P. Baruah, J. Geol. Soc. India, 2013, 82, 295–298 CrossRef CAS.
- G. Wang, J. Yang, J. Park, X. Gou, B. Wang, H. Liu and J. Yao, J. Phys. Chem. C, 2008, 112, 8192–8195 CAS.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, Nano Res., 2014, 8, 355–381 CrossRef.
- R. K. Boruah, B. K. Saikia, B. P. Baruah and N. C. Dey, J. Appl. Crystallogr., 2008, 41, 27–30 CrossRef CAS.
- B. K. Saikia, R. K. Boruah and P. K. Gogoi, J. Earth Syst. Sci., 2007, 116, 575–579 CrossRef CAS.
- B. K. Saikia, Int. J. Oil, Gas Coal Technol., 2010, 3, 362–373 CrossRef CAS.
- J. Yang, B. Pan, H. Li, S. Liao, D. Zhang, M. Wu and B. Xing, Environ. Sci. Technol., 2016, 50, 694–700 CrossRef CAS PubMed.
- X. Liu, F. Wang and S. Bai, Water Sci. Technol., 2015, 72, 2229–2235 CrossRef CAS PubMed.
- H. Lee, G. Sai-Anand, S. Komathi, A. I. Gopalan, S. W. Kang and K. P. Lee, J. Hazard. Mater., 2015, 283, 400–409 CrossRef CAS PubMed.
- L. Bo, X. Quan, S. Chen, H. Zhao and Y. Zhao, Water Res., 2006, 40(16), 3061–3068 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04392e |
| This journal is © The Royal Society of Chemistry 2016 |