Polyurethane foam functionalized with an AIE-active polymer using an ultrasonication-assisted method: preparation and application for the detection of explosives†
Zhu-Xin Fan,
Qing-Hua Zhao*,
Shi Wang,
Yu Bai,
Pei-Pei Wang,
Jin-Jin Li,
Zhi-Wei Chu and
Guo-Hua Chen*
College of Materials Science and Engineering, Huaqiao University, Xiamen 361021, P. R. China. E-mail: qhzhao@hqu.edu.cn; hdcgh@hqu.edu.cn; Fax: +86-592-6162225
First published on 26th February 2016
Abstract
PU foams modified with an AIE-active polymer (PSiTPE) with a Si–O and TPE backbone using an ultrasonication-assisted method have been prepared. The as-prepared foams show a high sensitive to PA solution and DNT vapor. Moreover, it could be re-used several times. It is a novel promising probe for the detection of explosives.
A tremendous amount of work has been put into detecting explosives,1 such as PA (2,4,6-trinitrophenol) and DNT (2,4-dinitrotoluene), due to the intrinsic risks to homeland security and environmental protection.
Typically, chemosensors based on fluorescence quenching with conjugated polymers have provided new reliable approaches, which are highly sensitive and cost-effective.2 Excited electrons or resonance energy transfer from electron-rich polymers (donors) to electron-deficient nitroaromatic compounds (acceptors) allows fluorescence quenching systems to be constructed.3 Unfortunately, traditional fluorescent polymers consist of aromatic group stacking, which may suffer from aggregation-caused quenching (ACQ) effects, impairing the detection results.4
Alternatively, aggregation induced emission (AIE) materials have received considerable attention due to their potential applications in the areas of optoelectronics5 and sensory systems.6 Tetraphenylethene (TPE) is the best AIE molecule, not only because of its easy preparation but also because it could be used to construct a large number of AIE materials, including small organic molecules and polymers.7 The fluorescence of TPE-based polymers can be effectively quenched by nitro explosives in solution or in the gas phase.8 By adopting this concept, numerous TPE-based polymers dispersed in good–poor pair solvents or casted into films as probes for explosive analysis have been extensively developed.9 However, there are few reports focused on using one detector for detecting explosives in aqueous media or vapor, simultaneously.
Nowadays, polyurethane sponges are widely used for absorbing both water and oils, or organic solvents, due to the 3D porous structure of PU and the facile method.10 Herein, through the attachment of AIE-active PSiTPE onto PU foams using an ultrasonication-assisted method, we prepared recyclable sensors for the detection of explosives in solution or vapor. Polysiloxane is a multifunctional backbone with good adhesion properties to various substrates and excellent thermal and chemical resistances.11 The Si–O moiety in PSiTPE contributes to connecting TPE groups as well as enhancing the stability of nanoparticles on PU foam.
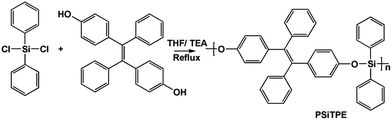
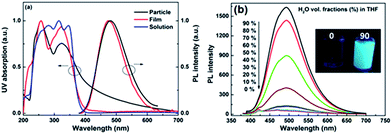
Scheme 1 illustrates the synthetic route to PSiTPE. The polymerization was conducted by nucleophilic aromatic substitution using triethylamine base with 80% yield. The detailed synthetic procedures and characterization data are presented in the ESI.† The number average molecular weight of PSiTPE is 4200 g mol−1 with a PDI of 1.5. The polymer is highly soluble in common organic solvents, such as tetrahydrofuran, dichloromethane, chloroform and toluene. The UV and PL spectra of PSiTPE in different states (solution, particles and film) are shown in Fig. 1(a). As depicted in Fig. 1(b), PSiTPE is non-emissive when dissolved in THF, but becomes luminescent at 492 nm with the gradual addition of H2O (>60%) into the THF solution, indicating that the polymer exhibits remarkable AIE features due to the TPE residue. The thermal stability of the polymer was recorded by thermogravimetric analysis (TGA) (Fig. S4†). Compared with the hydroxy monomer, the decomposition temperature (Td, corresponding to 5% weight loss) of PSiTPE shows a significant improvement from 118 °C to 263 °C, implying that the polymer possesses excellent thermal stability.
Ultrasonication-assisted manufacture is a convenient method, and has been applied in many fields, such as crushing, emulsification and activation of particles, because it can provide strong interactions between energy and matter.12 In the present study, when the polymer nanoparticles and PU foam were treated directly by ultrasonication, where blast waves with high speed and energy were formed pushing the particles towards the PU foam surface,13 the particles could be tightly adhered onto the PU foam skeleton. Therefore, PU-50, PU-100 and PU-150 were prepared by ultrasonication, and the detailed procedures can be found in the ESI.†
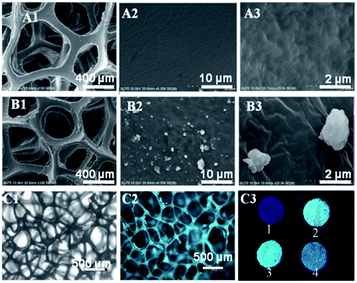
Before being treated by ultrasonication, we used an immersion method as a comparison. As depicted in Fig. 2 (3 and 4 in C3), the fluorescence intensity of the immersed PU foam declines drastically after it is put into ethanol with agitation for 30 min, suggesting that PSiTPE could be mostly washed away. The pure PU foam possesses a smooth surface, which can be seen from the SEM images in Fig. 2(A1)–(A3). In contrast, from Fig. 2(B1)–(B3), the modified PU foam surface was covered with polymer nanoparticles. Additionally, the non-luminous PU foam completely sparkles (1 and 2 in C3) after PSiTPE particles are anchored by ultrasonication treatment. As shown in Fig. 2(C1) and (C2), the foam skeleton with a bright bluish emission can be clearly observed from the fluorescence microscope photographs. The images of PU-50 and PU-150 are shown in Fig. S6.† Moreover, the as-prepared foam exhibits little change in the fluorescence intensity after 100 cyclic compression tests, which is favorable for its real application.
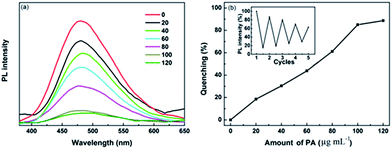
To investigate the potential application of the functionalized PU foams (PU-50, PU-100, PU-150) as detection probes for nitro explosives in solution, picric acid (PA) was selected as the analyte. When the sponge was put into a PA solution, the explosive molecules could spread inside the 3D lacunary framework, and then be seized by the nano-size particles. Subsequently, an effective fluorescence annihilation was recorded. Fig. 3(a) shows the PL spectra of PU-100 with the gradual addition of PA in methanol. Significant fluorescence quenching was observed with the introduction of the PA solution. A similar phenomenon was also observed for PU-50 and PU-150 (Fig. S7 and S8†). The quenching efficiencies are 72.7%, 85% and 77.5% (Fig. 3(b)) when the PA concentrations are 50, 100 and 150 μg mL−1, respectively. The plots of the quenching efficiency–PA concentration for PU-100 and PU-150 are linear. With regards to the Stern–Volmer formula, PU-100 and PU-150 give quenching constants (KSV) of 5616 and 4528 M−1, respectively. Moreover, PU-100 can be used multiple times after being washed with ethanol and dried at 60 °C in a vacuum oven. The results show that the initial fluorescence intensity was significantly retained (65%) after 4 recycles.
Inspired by this satisfactory result, we tried to apply the decorated foams to detect volatile explosives. Taking into consideration slow volatilization, ultralow loading concentrations (2.5, 5, 10 μg mL−1, THF/H2O = 1/9, volume) of particles were employed. The experiments were conducted by exposing the modified foams to saturated DNT vapor at room temperature. As shown in Fig. 4(a), fluorescence quenching was immediately observed and the fluorescence quenching of PU-2.5 reached 14.9% in 30 s, 77.3% in 90 s and 96% in 5 min. Meanwhile, the quenching efficiencies (Fig. 4(b)) of PU-5 and PU-10 in response to DNT vapor for 5 min were 78% and 46.5%, respectively, which are much lower than that of PU-2.5. In conjunction with various concentrations of the foams, for PU-2.5, gaseous DNT could easily diffuse into the porous cavities and then be trapped by the particles. For PU-5 and PU-10, the dense particles on the surface not only hamper the permeation of the DNT vapor but also hardly capture a sufficient number of explosive molecules for quenching. It is worth noting that, from Fig. S9,† PU-2.5 has great recycled sensitivity towards DNT. The exposed foams could be re-used by treating with hydrazine. Even after repeating 4 times, the quenching efficiency towards DNT vapor still remained about 63% within 5 min.
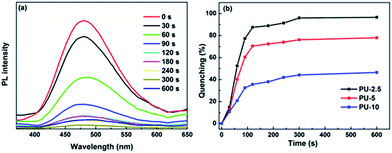 | ||
| Fig. 4 (a) Fluorescence quenching of PU-2.5 upon exposure to DNT saturated vapor; (b) time-dependent fluorescence quenching of PU-2.5, PU-5 and PU-10 upon exposure to saturated vapor of DNT. | ||
To shed light on the quenching mechanisms in the PA and DNT systems, charge transfer and resonance energy transfer were discussed. The energy levels (HOMO/LUMO) of the polymer, PA and DNT were evaluated. As listed in Fig. S10,† the LUMO of the polymer is much higher than that of PA and DNT, which facilitates excited electron transfer from the polymer to the analyte. Moreover, it was found that there is an overlap between the UV-vis absorption spectrum of PA and the emission spectrum of the polymer. Thus, resonance energy transfer may occur from PSiTPE to PA molecules, and neither exist in DNT.
In conclusion, we have successfully designed and synthesized an AIE-active polymer PSiTPE with a Si–O and TPE backbone. Furthermore, AIE-active nanoparticle functionalized PU foams with a high stability were prepared by ultrasonication, which deposited the polymer nanoparticles onto the flexible sponge surface. The 3D porous cavity structure of the foams was beneficial for the diffusion of explosive molecules. By adjusting the loading content, we have fabricated several kinds of decorated foams for detecting explosives in solution and vapor. For PA in methanol, the quenching efficiency of PU-100 reached 85% when the PA concentration was 100 μg mL−1. For DNT vapor, PU-2.5 gave 96% fluorescence quenching in 5 min. It is believed that the convenient preparation strategy can be extended to other AIE-based materials to develop more efficient probes for explosive detection both in solution and in the solid state. Further research on this issue is currently ongoing.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant no. 51202073 and 51373059) and the Startup Package Funding of Huaqiao University (11BS103). Qing-Hua Zhao thanks the Promotion Program for Young and Middle-aged Teacher in Science and Technology Research of Huaqiao University (ZQN-PY105) for financial support, the Instrumental Analysis Center of HUAQIAO UNIVERSITY for partial experimental data collection and Dr Zhao-hui Meng at Xia Men University for taking the fluorescence microscope photographs.Notes and references
- (a) S. Zahn and T. M. Swager, Angew. Chem., Int. Ed., 2002, 41, 4225 CrossRef; (b) I. A. Popov, H. Chen, O. N. Kharybin, E. N. Nikolaev and R. G. Cooks, Chem. Commun., 2005, 1, 953 Search PubMed; (c) J. W. Grate, Chem. Rev., 2008, 108, 726 CrossRef CAS PubMed.
- (a) H. H. Nguyen, X. Z. Li, N. Wang, Z. Y. Wang, J. J. Ma, W. Bock and D. G. Ma, Macromolecules, 2009, 42, 926 Search PubMed; (b) H. R. Nie, G. N. Sun, M. Zhang, M. Baumgarte and K. Mullen, J. Mater. Chem., 2012, 22, 2129 RSC; (c) B. W. Xu, Y. X. Xu, X. C. Wang, H. B. Li, X. F. Wu, H. Tong and X. L. Wang, Polym. Chem., 2013, 4, 5056 RSC; (d) A. Rose, Z. Zhu, C. F. Madigan, T. M. Swager and V. Bulovic, Nature, 2005, 434, 876 CrossRef CAS PubMed.
- (a) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 5321 CrossRef CAS; (b) H. R. Nie, Y. Zhao, M. Zhang, Y. G. Ma, M. Baumgarten and K. Mullen, Chem. Commun., 2011, 47, 1234 RSC; (c) J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 11864 CrossRef CAS.
- J. Liu, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2009, 109, 5799 CrossRef CAS PubMed.
- (a) Y. Liu, S. M. Chen, Y. Lam, P. Lu, R. T. K. Kwok, F. Mahtab, H. S. Kwok and B. Z. Tang, Chem. Mater., 2011, 23, 2536 CrossRef CAS; (b) J. Huang, Y. B. Jiang, J. Yang, R. L. Tang, N. Xie, B. Z. Tang and Z. Li, J. Mater. Chem. C, 2014, 2, 2028 RSC; (c) J. Huang, N. Sun, Y. Dong, R. Tang, P. Lu, P. Cai, Q. Li, D. Ma, J. Qin and Z. Li, Adv. Funct. Mater., 2013, 23, 2329 CrossRef CAS.
- (a) X. Qi, H. Li, J. W. Y. Lam, X. Yuan, J. Wei, B. Z. Tang and H. Zhang, Adv. Mater., 2012, 24, 4191 CrossRef CAS PubMed; (b) B. Wang, Y. Wang, J. Hua, Y. Jiang, J. Huang, S. Qian and H. Tian, Chem.–Eur. J., 2011, 17, 2647 CrossRef CAS PubMed; (c) J. Li, J. Liu, J. W. Y. Lam and B. Z. Tang, RSC Adv., 2013, 3, 8193 RSC; (d) D. Xu, X. Liu, R. Lu, P. Xue, X. Zhang, H. Zhou and J. Jia, Org. Biomol. Chem., 2011, 9, 1523 RSC.
- (a) W. Wu, S. Ye, R. Tang, L. Huang, Q. Li, G. Yu, Y. Liu, J. Qin and Z. Li, Polymer, 2012, 53, 3163 CrossRef CAS; (b) J. B. Shi, Y. M. Wu, S. Sun, B. Tong, J. G. Zhi and Y. P. Don, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 229 CrossRef CAS; (c) H. J. Yen, C. J. Chen and G. S. Liou, Chem. Commun., 2013, 49, 630 RSC.
- (a) R. Hu, J. L. Maldonado, M. Rodriguez, C. Deng, C. K. W. Jim, J. W. Y. Lam, M. M. F. Yuen, G. Ramos-Ortiz and B. Z. Tang, J. Mater. Chem., 2012, 22, 232 RSC; (b) A. Qin, J. W. Y. Lam, L. Tang, C. K. W. Jim, H. Zhao, J. Sun and B. Z. Tang, Macromolecules, 2009, 42, 1421–1424 CrossRef CAS; (c) X. M. Hu, Q. Chen, D. Zhou, J. Cao, Y. J. He and B. H. Han, Polym. Chem., 2011, 2, 1124 RSC.
- (a) H. Zhou, Q. Ye, W. T. Neo, J. Song, H. Yan, Y. Zong, B. Z. Tang, T. S. Andy Hor and J. W. Xu, Chem. Commun., 2014, 50, 13785 RSC; (b) M. X. Gao, Y. Wu, B. Chen, B. R. He, H. Nie, T. Y. Li, F. P. Wu, W. J. Zhou, J. Zhou and Z. J. Zhao, Polym. Chem., 2015, 6, 7641 RSC; (c) H. H. Nguyen, X. Li, N. Wang, Z. Y. Wang, J. Ma, W. J. Bock and D. Ma, Macromolecules, 2009, 42, 921 CrossRef CAS.
- (a) C.-F. Wang and S.-J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861 CrossRef CAS PubMed; (b) D. D. Nguyen, N.-H. Tai, S.-B. Lee and W.-S. Kuo, Energy Environ. Sci., 2012, 5, 7908 RSC; (c) C. F. Wang and S. J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 886 Search PubMed; (d) W. Chen, S. Li, C. Chen and L. Yan, Adv. Mater., 2011, 23, 5679 CrossRef CAS PubMed; (e) Q. Zhu and Q. Pan, ACS Nano, 2014, 8, 1402 CrossRef CAS PubMed.
- (a) Y. Liu, J. Ma, T. Wu, X. Wang, G. Huang, Y. Liu, H. Qiu, Y. Li, W. Wang and J. Gao, ACS Appl. Mater. Interfaces, 2013, 5, 10018 CrossRef CAS PubMed; (b) Q. Huang, G. A. Evmenenko, P. Dutta, P. Lee, N. R. Armstrong and T. J. Marks, J. Am. Chem. Soc., 2005, 127, 10227 CrossRef CAS PubMed; (c) D. M. Sun, Q. Fu, Z. J. Ren, W. Li, H. H. Li, D. G. Ma and S. K. Yan, J. Mater. Chem. C, 2013, 1, 5344 RSC; (d) D. M. Sun, Z. M. Yang, X. L. Sun, H. H. Li, Z. J. Ren, J. T. Liu, D. G. Ma and S. K. Yan, Polym. Chem., 2014, 5, 5046 RSC.
- (a) S. Doktycz and K. Suslick, Science, 1990, 247, 1067 CAS; (b) H. C. Shi, D. Shi, L. G. Yin, Z. H. Yang, S. F. Luan, J. F. Gao, J. W. Zha, J. H. Yin and R. K. Y. Li, Nanoscale, 2014, 6, 13748 RSC.
- (a) V. G. Pol, H. Grisaru and A. Gedanken, Langmuir, 2005, 21, 3635 CrossRef CAS PubMed; (b) I. Perelshtein, G. Applerot, N. Perkas, J. Grinblat, E. Hulla, E. Wehrschuetz-Sigl, A. Hasmann, G. Guebitz and A. Gedanken, ACS Appl. Mater. Interfaces, 2010, 2, 1999 CrossRef CAS PubMed; (c) J. Gao, M. Hu, Y. Dong and R. K. Y. Li, ACS Appl. Mater. Interfaces, 2013, 5, 7758 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra02134d |
| This journal is © The Royal Society of Chemistry 2016 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: