Fluorescence staining of salicylaldehyde azine, and applications in the determination of potassium tert-butoxide†
Abstract
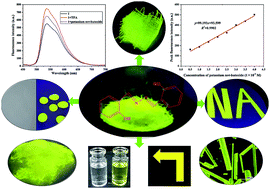
Salicylaldehyde azine (1), with an aggregation-induced emission (AIE) function, was synthesized from salicylaldehyde and characterized by Fourier transform infrared spectroscopy (FT-IR), nuclear magnetic resonance (NMR), high resolution mass spectrometry (HRMS), and X-ray single crystal diffraction. Compound 1 exhibited high thermal stability and good light-emitting behavior in the solid state. It could be preferably used to dye cellulose and KBr. No obvious changes in fluorescence intensity occurred when compound 1 was dissolved in different pH buffer solutions. The addition of TFA led to slight fluorescence quenching, and the fluorescence intensity gradually increased with increasing potassium tert-butoxide solution concentrations. The fluorescence intensity of compound 1 showed a good linear relationship with the concentration of potassium tert-butoxide (0.5–4.0 × 10−4 M), y = 99.193x + 93.599, R2 = 0.9902; the limit of detection (LOD) was 1.07 × 10−7 M. It could measure the content of potassium tert-butoxide with a relative standard deviation (RSD) value of 1.5%.

 Please wait while we load your content...
Please wait while we load your content...