Fluorophores based on a minimal thienylthiazole core: towards multifunctional materials with solid state red emissions, solvatochromism and AIE behaviour†
Rakesh Radhakrishnan and
Kumaran G. Sreejalekshmi*
Department of Chemistry, Indian Institute of Space Science and Technology, Valiamala Post, Thiruvananthapuram – 695 547, India. E-mail: sreeja@iist.ac.in; sreejalekshmi@gmail.com
First published on 24th March 2016
Abstract
We introduce 5-(thiophen-2-yl)-1,3-thiazole [5-TPTZ] as a novel core for the design of luminescent organic materials. Donor–acceptor–donor–acceptor (D–A–D–A) tetrads based on [5-TPTZ] exhibited solid state red emission, positive solvatochromism, large Stokes shift, and aggregation induced emission (AIE) behaviour. The observed photophysical properties of these multifunctional materials were elucidated by crystal structure analysis, computational studies and molecular dynamics (MD) simulations.
Luminescent organic material (LOM) research continues to be topical and seeking new candidates with enhanced properties, ease of synthesis and processability. Although a myriad of LOMs are reported with emissions in blue,1 green,2 yellow,3 orange4 and red regions,5 a majority turn out be non-luminous in the condensed state.6 Thus concentration quenching or aggregation caused quenching (ACQ) impeded LOMs' practical applications considerably until Tang et al. reported on the anti-ACQ photophysical phenomenon, aggregation induced emission (AIE)7 which spurred prolific research and expanded the horizon for technological applications.8 However, the limited availability of expandable cores and associated synthetic challenges9 still hinder the development of solid state red emitters (SSREs), highly sought after for a multitude of applications ranging from optoelectronics,10 chemical sensors,11 fluorescent probes and biomedical imaging.12 Currently, the design of SSREs predominantly focus on π conjugated skeletons, either in polycyclic aromatic hydrocarbon or in donor (D)–acceptor (A) systems,13 whereas novel core systems is need of the hour.14 From the heterocyclic tool box, thiophene (D) and thiazole (A) systems have been explored separately5b,15 as well as in combinations.16 However, till date, the potential of archetypal thienylthiazole core has not been explored in SSREs and for the first time, we introduce 5-(thiophen-2-yl)-1,3-thiazole [5-TPTZ] as a promising core for the design of LOMs with tunable and exciting luminescence behaviour. To the best of our knowledge, 5-TPTZ affords the smallest molecular systems among SSREs reported ever which are synthetically accomplished and systematically studied.
The synthesis of D–A–D–A tetrads based on 5-TPTZ core was realised following a general and efficient route developed in our group17 by reacting aroylthioureas with 2-(bromomethyl)-5-nitrothiophene in a tandem nucleophilic reaction under mild conditions (ESI†). Apart from the alternating D–A units, yet another interesting attribute of our novel core is that it accommodates a tunable handle at C-4 of the thiazole ring system, a contribution from an aroyl/heteroyl chloride, whereby the tetrads would acquire flexibility of tuning optical and electronic properties. Herein, we selected three systems viz.; 5-TPTZ-1, 5-TPTZ-2 and 5-TPTZ-3 (Fig. 1) which were constructed with three different D groups at C-2 of thiazole ring by varying the secondary amine component during the synthesis of the required aroylthiourea and were isolated as red solids from the reaction mixture. The unique configuration of the minimal architecture push–pull tetrads, with virtually multidirectional intramolecular charge transfer (ICT) capabilities (Fig. 1) which would arise from the D/A groups on C-4, encouraged us to investigate their photophysical properties. In this brief communication, we present on the solid and solution state luminescence studies as well as AIE behaviour in the selected systems.
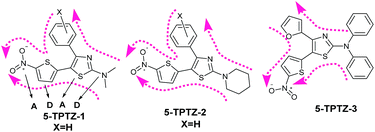 | ||
| Fig. 1 Chemical structures of SSREs 5-TPTZ-1–3 and the plausible directions of intramolecular charge transfer. | ||
The absorption spectra of 5-TPTZ-1, 5-TPTZ-2 and 5-TPTZ-3 recorded in tetrahydrofuran (THF) showed λmax values of 473, 476 and 472 nm respectively and the corresponding emissions were in the near-red region with λ values 614, 603 and 599 nm (Fig. 2a and b). All the molecules exhibited wavelength independent emission as evident from the excitation and absorption spectra (Fig. S1, ESI†). We could successfully reproduce the absorption wavelength of these molecules by density functional theory (DFT) calculations (Table S3, ESI†). The quantum yield of 5-TPTZ-1, 5-TPTZ-2 and 5-TPTZ-3 measured in THF were 0.02, 0.13 and 0.24 respectively. The unusual large Stokes shift (SS > 120 nm) was elucidated on the basis of difference in ground and excited state geometries as predicted by detailed computational studies.‡ Furthermore, strong push–pull nature of the tetrad leading to intramolecular charge transfer (ICT) would also justify large SS values. To attest the ICT behaviour, we carried out frontier molecular orbital (FMO) analysis, whereby highest occupied molecular orbital (HOMO) of the molecules were found delocalized on whole of the molecular structure and lowest unoccupied molecular orbital (LUMO) predominantly localized on the nitro acceptor on thiophene C-5 suggesting a charge transfer from donor to acceptor (Fig. 3). This would further hint the potential of the systems to exhibit solvent polarity dependent luminescence properties for which the solubility of the systems in a wide range of solvents become critical. The selected molecules were found to be soluble in a broad range of organic solvents and hence we proceeded with their solution state luminescence studies.
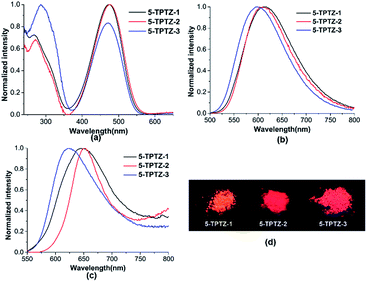 | ||
| Fig. 2 (a) UV/Vis absorption spectra (b) emission spectra in THF (c = 10−5 mol L−1) (c) emission spectra recorded with thin films (d) photograph of solid samples under UV illumination, 365 nm. | ||
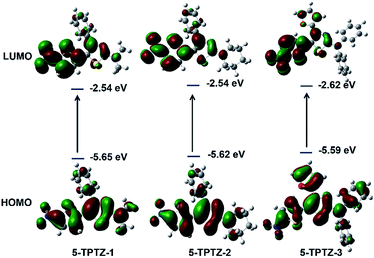 | ||
| Fig. 3 FMO diagrams from computational studies (calculated at PBE0/6-31G(d,p)) level in THF suggestive of probable solvatochromic properties. | ||
In line with our expectations, we noticed 5-TPTZ-1, 5-TPTZ-2 and 5-TPTZ-3 to be sensitive to perturbations in the solvent environment and exhibited positive solvatochromism both in absorption and emission spectra. For example, the emission maximum centered at 533 nm for 5-TPTZ-1 in hexane underwent a shift to 614 nm in THF (SS 141 nm, 4855 cm−1) and 647 nm in acetonitrile (SS 168 nm, 5397 cm−1). The positive solvatochromism is explained by the differences in the ground and excited state geometries and the stabilizing effect of polar solvents on the excited state.‡ The fluorescence solvatochromism was better explained by Lippert–Mataga plot.18 The plot of Stokes shift (cm−1) versus solvent orientation polarizability furnished a linear fit (ESI†) and we anticipate that this sensitivity to solvent polarity could be an attribute arising from unique 5-TPTZ core and hence indicative of vast scope for expanding the core to afford environment sensitive fluorophores19 tailored for specific applications, studies pertaining to which are currently being pursued in our laboratories.
Strikingly, these novel D–A–D–A tetrads (5-TPTZ-1–3) under UV illumination were found to be red emissive (Fig. 2c and d) and we were excited to proceed with the solid state luminescence studies on these smallest systems ever reported with similar behaviour. Emissions from the solid samples (thin films) were observed at λ values 649 nm (ϕ = 0.07) for 5-TPTZ-1, 650 nm (ϕ = 0.05) for 5-TPTZ-2, and 622 nm (ϕ = 0.05) for 5-TPTZ-3 and with solid state fluorescence quantum yield as indicated in the parenthesis. Considering the minimal architecture contributing to the encouraging results, we successfully demonstrated the potential of 5-TPTZ as a novel core in the family of SSREs, thus addressing two major challenges in LOMs research viz.; synthetic hurdle and processability.
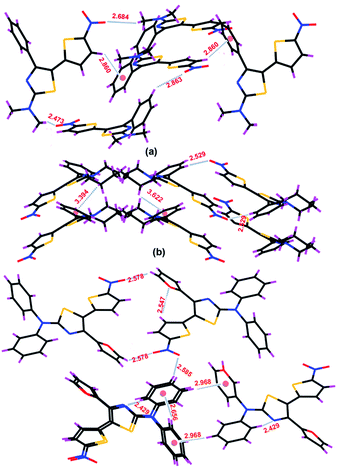
To rationalize the observed solid state emissions, we examined the crystal landscapes using single crystal XRD (Fig. 4 and ESI†). Incidentally, each of the systems crystallised in a unique space group – 5-TPTZ-1 (monoclinic; P21/n), 5-TPTZ-2 (orthorhombic; Pbca) and 5-TPTZ-3 (triclinic; P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ). In 5-TPTZ-1 and 5-TPTZ-1, thiazole and thiophene rings adopted trans configuration, whereas in 5-TPTZ-3 a cis configuration was noticed, probably due to the preference of furan ring oxygen for an intramolecular H-bond (2.547 Å) with thiophene C2–H. The dihedral angles between thiazole and thiophene (S1C4C5S2 177.49° in 5-TPTZ-1, S1C1C4S2 147.52° in 5-TPTZ-2 and S1C4C5S 225.40° in 5-TPTZ-3), and the torsional angle imparted by C4 substituent on thiazole ring, all contribute to non-planarity and hence minimise possibilities of non-radiative pathways. Moreover, analyses of supramolecular structures threw light on the critical role of multiple short interactions (C–H⋯π interaction, H-bonding) in imparting structural rigidity to the molecular system, which further bring significance to our core design and relative positioning of the substituents.
). In 5-TPTZ-1 and 5-TPTZ-1, thiazole and thiophene rings adopted trans configuration, whereas in 5-TPTZ-3 a cis configuration was noticed, probably due to the preference of furan ring oxygen for an intramolecular H-bond (2.547 Å) with thiophene C2–H. The dihedral angles between thiazole and thiophene (S1C4C5S2 177.49° in 5-TPTZ-1, S1C1C4S2 147.52° in 5-TPTZ-2 and S1C4C5S 225.40° in 5-TPTZ-3), and the torsional angle imparted by C4 substituent on thiazole ring, all contribute to non-planarity and hence minimise possibilities of non-radiative pathways. Moreover, analyses of supramolecular structures threw light on the critical role of multiple short interactions (C–H⋯π interaction, H-bonding) in imparting structural rigidity to the molecular system, which further bring significance to our core design and relative positioning of the substituents.
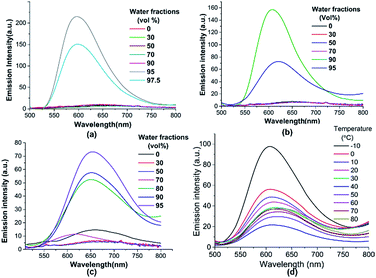
In view of the significance of AIE and aggregation induced emission enhancement (AIEE) properties for few practical applications,8f we next studied the emission properties of aggregates of 5-TPTZ-1–3 generated by varying water fractions (fw) in acetonitrile solution whereupon a red shifted, low intense emission possibly due to the formation of a twisted ICT state was noted. At higher fw, emission intensity increased suddenly with a blue shift. For 5-TPTZ-1, a 21 fold increase in emission intensity was achieved at fw of 95% whereas there was 17 and 5 fold increase for 5-TPTZ-2 and 5-TPTZ-3 respectively at a fw of 90% (Fig. 5a–c). To ascertain the role of restriction of intramolecular rotation (RIR) within the tetrad, which can be directly correlated to molecular design, we carried out temperature dependant emission studies.20
Emission spectrum of 5-TPTZ-1 in acetonitrile–water mixture with fw of 95% is shown in Fig. 5d. A decrease of temperature from 80 °C to −10 °C was associated with intensity enhanced emission and a 4.5 fold enhancement in intensity was achieved at temperature of −10 °C, which could be a direct consequence of RIR and thus blockage of non-radiative pathways.
To understand the time dependent evolution of the aggregates, we conducted molecular dynamics (MD) simulations using Desmond MD program.21 MD simulations of 5-TPTZ-1 in water revealed the formation of aggregates in nanosecond time scale (Fig. 6). In a 10 ns calculation, we could observe clustering of molecules to form aggregates which were further stabilized by H-bonding interactions with water molecules. The radial distribution function (RDF) between 5-TPTZ-1 and water molecules (ESI†) exhibited a shoulder around 3.6 Å which was attributed to the H-bond between nitro group and water molecules. In the molecular cluster, the 5-TPTZ-1 molecules adopted an orientation where nitro groups dominated the periphery of the aggregates which consecutively resulted in a shell of water molecules (ESI†) enclosing the aggregate and hence stabilising it. On the contrary, when we performed MD simulation with DMSO in which 5-TPTZ-1 is highly soluble, the molecules were found to be randomly distributed throughout the box volume and no aggregate formation was observed (ESI†). Thus we have effectively applied MD studies to validate aggregate formations§ in 5-TPTZ-1 and encouraged by the preliminary results, we are expanding our studies to more systems and expect that our studies will boost more of such simulations which are currently scarce in AIE materials research.22
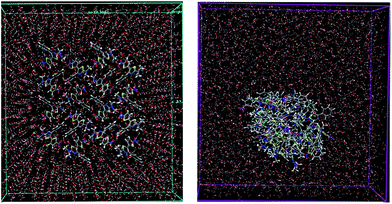 | ||
| Fig. 6 MD simulation of 5-TPTZ-1 in water. Distribution of molecules at the start of simulations (left) aggregate formation after 10 ns (right). | ||
In summary, we have successfully contributed to LOMs research by introducing 5-(thiophen-2-yl)-1,3-thiazole [5-TPTZ] as a novel and expandable core for minimal architecture SSREs. D–A–D–A tetrads 5-TPTZ-1, 5-TPTZ-2 and 5-TPTZ-3 based on the core displayed a broad spectrum of exciting photophysical properties in addition to solid state red emission including solvatochromism, large Stokes shift, and AIE behaviour. Using computational tools, crystal analysis, solution and solid state luminescence studies and MD simulations we explained the observed photophysical properties. Considering the simple synthesis, solution processability and multiple tunable sites, this novel fluorophore family could be considered as a leap forward in the field of multifunctional molecular materials. It is noteworthy that, these new candidates in the family of SSREs address critical challenges in the field related to synthetic routes and processability and our laboratories are continuing with the molecular design around the core for improved photophysical properties.
Acknowledgements
RR acknowledges IIST for financial assistance. We thank IISER-TVM, NIIST-TVM and SAIF-IITM for the spectral data and single crystal XRD measurements.Notes and references
- (a) K.-T. Wong, Y.-Y. Chien, R.-T. Chen, C.-F. Wang, Y.-T. Lin, H.-H. Chiang, P.-Y. Hsieh, C.-C. Wu, C. H. Chou and Y. O. Su, J. Am. Chem. Soc., 2002, 124, 11576 CrossRef CAS PubMed; (b) J. Luo, Y. Zhou, Z.-Q. Niu, Q.-F. Zhou, Y. Ma and J. Pei, J. Am. Chem. Soc., 2007, 129, 11314 CrossRef CAS PubMed; (c) Y.-H. Kim, D.-C. Shin, S.-H. Kim, C.-H. Ko, H.-S. Yu, Y.-S. Chae and S.-K. Kwon, Adv. Mater., 2001, 13, 1690 CrossRef CAS.
- (a) S.-Y. Ku, L.-C. Chi, W.-Y. Hung, S.-W. Yang, T.-C. Tsai, K.-T. Wong, Y.-H. Chen and C.-I. Wu, J. Mater. Chem., 2009, 19, 773 RSC; (b) C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS.
- (a) W. Z. Yuan, Y. Gong, S. Chen, X. Y. Shen, J. W. Lam, P. Lu, Y. Lu, Z. Wang, R. Hu and N. Xie, Chem. Mater., 2012, 24, 1518 CrossRef CAS; (b) R. Flamholc, D. Plażuk, J. Zakrzewski, R. Metivier, K. Nakatani, A. Makal and K. Woźniak, RSC Adv., 2014, 4, 31594 RSC.
- (a) D. M. D'Souza, C. Muschelknautz, F. Rominger and T. J. Müller, Org. Lett., 2010, 12, 3364 CrossRef PubMed; (b) R. M. Adhikari, D. C. Neckers and B. K. Shah, J. Org. Chem., 2009, 74, 3341 CrossRef CAS PubMed.
- (a) T. Ozdemir, S. Atilgan, I. Kutuk, L. T. Yildirim, A. Tulek, M. Bayindir and E. U. Akkaya, Org. Lett., 2009, 11, 2105 CrossRef CAS PubMed; (b) W. Li, W. Lin, J. Wang and X. Guan, Org. Lett., 2013, 15, 1768 CrossRef CAS PubMed.
- (a) A. Wakamiya, T. Taniguchi and S. Yamaguchi, Angew. Chem., 2006, 45, 3170 CrossRef CAS PubMed; (b) S. Mukherjee and P. Thilagar, Proc. Natl. Acad. Sci., India, Sect. A, 2014, 84, 131 CrossRef; (c) M. Shimizu and T. Hiyama, Chem.–Asian J., 2010, 5, 1516 CrossRef CAS PubMed.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740 RSC.
- (a) D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed; (b) Y. Hong, J. W. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (c) Y. Hong, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (d) R. T. Kwok, C. W. Leung, J. W. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228 RSC; (e) J. Mei, Y. Hong, J. W. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429 CrossRef CAS PubMed; (f) J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718 CrossRef CAS PubMed.
- W. Qin, J. W. Lam, Z. Yang, S. Chen, G. Liang, W. Zhao, H. S. Kwok and B. Z. Tang, Chem. Commun., 2015, 51, 7321 RSC.
- (a) J. Zhang, Y. Yang, C. He, Y. He, G. Zhao and Y. Li, Macromolecules, 2009, 42, 7619 CrossRef CAS; (b) H.-C. Yeh, S.-J. Yeh and C.-T. Chen, Chem. Commun., 2003, 20, 2632 RSC; (c) Y. Wang, J. Zhou, X. Wang, X. Zheng, Z. Lu, W. Zhang, Y. Chen, Y. Huang, X. Pu and J. Yu, Dyes Pigm., 2014, 100, 87 CrossRef CAS; (d) W. C. Wu, H. C. Yeh, L. H. Chan and C. T. Chen, Adv. Mater., 2002, 14, 1072 CrossRef CAS; (e) Z. Ning, Z. Chen, Q. Zhang, Y. Yan, S. Qian, Y. Cao and H. Tian, Adv. Funct. Mater., 2007, 17, 3799 CrossRef CAS.
- (a) J. Fan, M. Hu, P. Zhan and X. Peng, Chem. Soc. Rev., 2013, 42, 29 RSC; (b) Z. Fang, K.-Y. Pu and B. Liu, Macromolecules, 2008, 41, 8380 CrossRef CAS; (c) H. Luo, S. Chen, Z. Liu, C. Zhang, Z. Cai, X. Chen, G. Zhang, Y. Zhao, S. Decurtins and S. X. Liu, Adv. Funct. Mater., 2014, 24, 4250 CrossRef CAS.
- (a) F. Hu, Y. Huang, G. Zhang, R. Zhao, H. Yang and D. Zhang, Anal. Chem., 2014, 86, 7987 CrossRef CAS PubMed; (b) G. Liu, D. Chen, L. Kong, J. Shi, B. Tong, J. Zhi, X. Feng and Y. Dong, Chem. Commun., 2015, 51, 8555 RSC; (c) R. Weissleder and M. J. Pittet, Nature, 2008, 452, 580 CrossRef CAS PubMed; (d) Q. Zhao, K. Li, S. Chen, A. Qin, D. Ding, S. Zhang, Y. Liu, B. Liu, J. Z. Sun and B. Z. Tang, J. Mater. Chem., 2012, 22, 15128 RSC; (e) X. Q. Zhang, X. Y. Zhang, B. Yang, Y. Zhang and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 3600 CrossRef CAS PubMed.
- (a) S. J. Lord, H.-l. D. Lee, R. Samuel, R. Weber, N. Liu, N. R. Conley, M. A. Thompson, R. J. Twieg and W. Moerner, J. Phys. Chem. B, 2009, 114, 14157 CrossRef PubMed; (b) H. Meier, Angew. Chem., Int. Ed., 2005, 44, 2482 CrossRef CAS PubMed; (c) T. Beppu, K. Tomiguchi, A. Masuhara, Y. J. Pu and H. Katagiri, Angew. Chem., Int. Ed., 2015, 54, 7332 CrossRef CAS PubMed; (d) R. Gompper and H. U. Wagner, Angew. Chem., Int. Ed. Engl., 1988, 27, 1437 CrossRef; (e) C.-T. Chen, Chem. Mater., 2004, 16, 4389 CrossRef CAS; (f) M. Kim, D. R. Whang, J. Gierschner and S. Y. Park, J. Mater. Chem. C, 2015, 3, 231 RSC.
- A. C. Shaikh, D. S. Ranade, S. Thorat, A. Maity, P. P. Kulkarni, R. G. Gonnade, P. Munshi and N. T. Patil, Chem. Commun., 2015, 51, 16115 RSC and references therein.
- (a) A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed; (b) S. Wen, J. Liu, M. Qiu, Y. Li, D. Zhu, C. Gu, L. Han and R. Yang, RSC Adv., 2015, 5, 5875 RSC; (c) Y. Kubota, S. Tanaka, K. Funabiki and M. Matsui, Org. Lett., 2012, 14, 4682 CrossRef CAS PubMed; (d) K. Anthony, R. G. Brown, J. D. Hepworth, K. W. Hodgson, B. May and M. A. West, J. Chem. Soc., Perkin Trans. 2, 1984, 2111 RSC.
- (a) G. Liu, S. Pu and X. Wang, Tetrahedron, 2010, 66, 8862 CrossRef CAS; (b) S. P. Mishra, A. K. Palai, A. Kumar, R. Srivastava, M. N. Kamalasanan and M. Patri, Macromol. Chem. Phys., 2010, 211, 1890 CrossRef CAS; (c) S. Ando, J.-I. Nishida, Y. Inoue, S. Tokito and Y. Yamashita, J. Mater. Chem., 2004, 14, 1787 RSC.
- R. Radhakrishnan and K. G. Sreejalekshmi, New J. Chem., 2016 10.1039/c6nj00418k.
- (a) E. Lippert, Zeitschrift für Naturforschung A, 1955, 10, 541 CrossRef; (b) N. Mataga, Y. Kaifu and M. Koizumi, Bull. Chem. Soc. Jpn., 1956, 29, 465 CrossRef CAS.
- Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563 RSC.
- R. Hu, E. Lager, A. Aguilar-Aguilar, J. Liu, J. W. Lam, H. H. Sung, I. D. Williams, Y. Zhong, K. S. Wong and E. Pena-Cabrera, J. Phys. Chem. C, 2009, 113, 15845 CAS.
- (a) Desmond Molecular Dynamics System, version 4.3, D. E. Shaw Research, New York, NY, 2015 Search PubMed; (b) Maestro-Desmond Interoperability Tools, version 4.3, Schrödinger, New York, NY, 2015 Search PubMed.
- G. Sun, M.-G. Ju, H. Zang, Y. Zhao and W. Liang, Phys. Chem. Chem. Phys., 2015, 17, 24438 RSC and references therein.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. CCDC 1424468, 1440185 and 1440186. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra00672h |
| ‡ The detailed computational studies on thienylthiazole core will appear elsewhere. |
| § MD simulations for 10 ns of 5-TPTZ-1 is available as video in AVI format. |
| This journal is © The Royal Society of Chemistry 2016 |