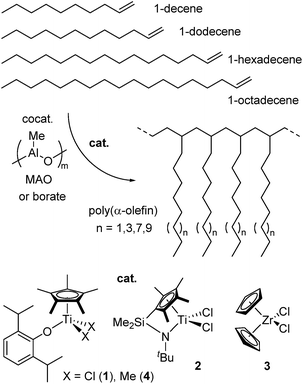
Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher α-olefins catalysed by aryloxo-modified half-titanocenes†
Kotohiro Nomura*,
Sarntamon Pengoubol and
Wannida Apisuk
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioji, Tokyo 192-0397, Japan. E-mail: ktnomura@tmu.ac.jp; Fax: +81-42-677-2547; Tel: +81-42-677-2547
First published on 2nd February 2016
Abstract
Polymerisation of 1-decene, 1-dodecene, 1-hexadecene, and 1-octadecene by Cp*TiCl2(O-2,6-iPr2C6H3) (1) – MAO catalysts yielded high molecular weight polymers with unimodal molecular weight distributions, and the Mn values in the resultant polymers were higher than those prepared by linked half-titanocene, [Me2Si(C5Me4) (NtBu)]TiCl2 and ordinary zirconocenes (Cp2ZrCl2 etc.); Cp*TiMe2(O-2,6-iPr2C6H3) – [Ph3C][B(C6F5)4] catalyst showed higher catalytic activities, affording ultrahigh molecular weight polymers (Mn = >1.07 × 106).
Introduction
Polyolefins produced by metal catalysed olefin polymerisation are important synthetic polymers in industry, and synthesis of new polymers with specified functions is an attractive research subject. Design of molecular catalysts has been considered to play an important role for synthesis of new polymers,1,2 and the recent progress offers new possibilities.1–6 In contrast to successful applications of isotactic polypropylene as a crystalline material, the use of amorphous poly(α-olefin)s (APAOs) has been shown less attention due to their inherent stickiness and softness. However, APAOs are used in hot melt applications due to their high melt flow rate with low density; these are known to improve adhesion on wood and PP, cohesion and even improve the free-flowing ability of the APAO granules.7In general, as described below, polymerisation of α-olefin (1-hexene, 1-octene etc.) by ordinary metallocene catalysts gave oligomers (Mn = ca. 4000),8 and several examples8–13 were known for synthesis of (ultra)high molecular weight poly(1-hexene)s by using [2,2-(O-4-Me-6-tBu-C6H3)2S]TiCl2 (in the presence of specified modified MMAO),9 or (C5HMe4)2HfCl2 (under ultrahigh pressure);10 some examples by the others afforded rather high molecular weight polymers.14 It was reported by us that polymerisation of α-olefin (1-hexene, 1-octene) by Cp*TiCl2(O-2,6-iPr2C6H3) (1) – MAO catalyst afforded high molecular weight polymers11 that were rather higher than those prepared by CpTiCl2(N = tBu2) – MAO catalyst,12 and the others.14 Moreover, we reported that polymerisation of 1-hexene by Cp*TiMe2(O-2,6-iPr2C6H3) – borate catalyst afforded ultrahigh molecular weight poly(1-hexene)s (Mn = >1.0 to 1.9 × 106);13 the polymerisation proceeded in a quasi-living manner under certain optimised conditions.13b
In this paper, we thus report our explored results for polymerisation of higher α-olefins (1-dodecene, 1-hexadecene, 1-octadenece) using Cp*TiX2(O-2,6-iPr2C6H3) (X = Cl, Me) – cocatalyst systems including comparison with those by [Me2Si(C5Me4)(NtBu)]TiCl2 (ordinary linked half-titanocene), Cp2ZrCl2 (ordinary metallocene).15 In particular, we wish to demonstrate that synthesis of ultrahigh molecular weight (brush like) polymers by polymerisation of 1-dodecene has been achieved especially by using Cp*TiMe2(O-2,6-iPr2C6H3) – borate catalyst Scheme 1.15
Results and discussion
Polymerisation of 1-decene (DC), 1-dodecene (DD), 1-hexadecene (HD), 1-octadecene (OD) by Cp*TiX2(O-2,6-iPr2C6H3) (X = Cl, Me), [Me2Si(C5Me4)(NtBu)]TiCl2, Cp2ZrCl2
It turned out that, as reported previously (in the 1-hexene and 1-octene polymerization),11 polymerisation of 1-decene (DC), 1-dodecene (DD) using Cp*TiCl2(O-2,6-iPr2C6H3) (1) – MAO catalyst proceeded with high catalytic activities affording high molecular weight polymers with uniform molecular weight distributions (Mn = 4.35 to 7.88 × 105, Mw/Mn = 1.23–1.84, shown in Table S1 in the ESI†);16,17 no significant effects of number of side chain (from 1-hexene to 1-dodecene) toward the Mn values were observed. The activity was affected by the Al/Ti molar ratios (shown in Table S1†),16 but significant differences in the Mn values were not observed by varying the Al/Ti molar ratios. The results could suggest that β-hydrogen elimination would be a major chain transfer rather than transfer to Al in this catalysis.Polymerisations of 1-octene (OC), 1-decene (DC), 1-dodecene (DD), 1-hexadecene (HD), and 1-octadecene (OD) using 1, [Me2Si(C5Me4)(NtBu)]TiCl2 (2), Cp2ZrCl2 (3) were conducted in the presence of MAO (α-olefin 5.0 mL, 25 °C, 30 min), and the results are summarised in Table 1. It turned out that complex 1 showed higher catalytic activities than 2 in the polymerisations of OC, DC, DD under the same conditions (runs 1–5, 9–12). For example, the activity by 1 in DD polymerisation (TON 4120 after 30 min, run 5) was higher than that by 2 (2060, run 12), and the Mn value in the resultant polymer by 1 was higher than that by 2 [Mn = 5.25 × 105 (by 1, run 5) vs. 3.51 × 105 (by 2, run 12)]; the similar trend was observed in these polymerisations. Moreover, the observed activities by 1 in 1-octene and 1-decene polymerisations were higher than those previously reported by [2,2-(O-4-Me-6-tBu-C6H3)2S]TiCl2 (activity 300 kg mol−1-Ti h in OC polymerisation),9c [O-2-tBu-6-(PhN = CH)C6H3]TiCl2 (activity ca. 1480 kg mol−1-Ti h in DC polymerisation).8 The Mn values in the resultant poly(DC)s, poly(DD)s prepared by 1 were higher than those by 2. However, no significant difference in both the activity and the Mn values in the resultant polymers were observed in HD, OD polymerisation using 1,2 – MAO catalysts. In contrast, the reactions with Cp2ZrCl2 (3) afforded low molecular weight oligomers with high catalytic activities, as observed in the polymerisation by rac-[Et(indenyl)2]ZrCl2 – borate catalyst.8 The observed activities by 3 were higher than those reported previously by the bis(indenyl) analogue.8 It is thus clear that catalysts 1,2 are effective for synthesis of high molecular weight polymers with unimodal molecular weight distributions (by 1, Mn = 2.27 to 5.25 × 105, Mw/Mn = 1.35–1.49, runs 5–8). The resultant polymers possessed atactic stero-regularity (and are amorphous materials),18,19 which possess melting temperatures (Tm) in poly(DD), poly(HD) due to their longer side chains (side chain crystallization); the Tm value increased upon increasing number of the methylene units.18
| Run | Cat. | α-Olefin | Yield/mg | TON | Activityb | Mnc | Mw/Mnc | Tmd/°C |
|---|---|---|---|---|---|---|---|---|
| a Conditions: complex 1.0 μmol, α-olefin 5.0 mL, d-MAO (prepared by removing toluene and AlMe3 from ordinary MAO) 2.0 mmol, 25 °C, 30 min.b TON (turnover number) = (mmol of α-olefin reacted)/(mmol-Ti).c Activity = kg-polymer/mol-Ti h.d GPC data THF vs. polystyrene standards. | ||||||||
| 1 | 1 | OC | 1117 | 9950 | 2240 | 501![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.42 | |
| 2 | 1 | OC | 1070 | 9530 | 2140 | 492![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.49 | |
| 3 | 1 | DC | 922 | 6570 | 1840 | 574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.84 | |
| 4 | 1 | DC | 904 | 6450 | 1810 | 435![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.78 | |
| 5 | 1 | DD | 694 | 4120 | 1390 | 525![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.35 | −24 |
| 6 | 1 | HD | 234 | 1040 | 470 | 389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.35 | 26 |
| 7 | 1 | OD | 289 | 1140 | 580 | 227![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.42 | 42 |
| 8 | 1 | OD | 285 | 1130 | 570 | 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.49 | |
| 9 | 2 | OC | 540 | 4810 | 1080 | 292![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.64 | |
| 10 | 2 | DC | 430 | 3070 | 860 | 301![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.47 | |
| 11 | 2 | DC | 410 | 2920 | 820 | 231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.82 | |
| 12 | 2 | DD | 346 | 2060 | 690 | 351![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.33 | −24 |
| 13 | 2 | HD | 258 | 1150 | 510 | 291![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.57 | 26 |
| 14 | 2 | HD | 254 | 1130 | 510 | 303![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.49 | |
| 15 | 2 | OD | 215 | 852 | 430 | 245![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.70 | 42 |
| 16 | 3 | OC | 2395 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4790 | 4100 | 1.55 | |
| 17 | 3 | DC | 2432 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4860 | 4400 | 1.56 | |
| 18 | 3 | DC | 2646 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
5290 | 4000 | 1.62 | |
| 19 | 3 | DD | 2594 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
5190 | 5000 | 1.57 | |
| 20 | 3 | HD | 2501 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
5000 | 4700 | 1.35 | 26 |
| 21 | 3 | OD | 1105 | 4380 | 2210 | 6600 | 1.46 | 42 |
Polymerisation of 1-octene (OC), 1-dodecene (DD) using Cp*TiMe2(O-2,6-iPr2C6H3) (4) – AliBu3 – [Ph3C][B(C6F5)4] catalyst
It should be noted that Cp*TiMe2(O-2,6-iPr2C6H3) (4) – AliBu3 – [Ph3C][B(C6F5)4] catalyst afforded ultrahigh molecular weight polymers with uniform molecular weight distributions in 1-dodecene (DD) polymerisation at −30 °C (Mn = 9.46 to 14.5 × 105, Mw/Mn = 1.62–1.99, runs 27–30, Table 2). The observed activities in the DD polymerisation (at −30 °C) by 4 – borate catalyst were higher than those by 1 – MAO catalyst (at 25 °C, shown in Table 1). Moreover, as exemplified in OC polymerisation (runs 22–26) as well as demonstrated by 1-hexene polymerisation under certain conditions,13b the Mn values increase upon increasing the TON values (polymer yields, conversions). The resultant polymers possessed atactic stero-regularity [amorphous in poly(1-octene)s (glass transition temperature (Tg) = −62 °C) and only melting temperature (−26 °C) was observed in poly(1-dodecene), shown in ESI, Fig. S4†],18 as observed in the polymerisation using 1 – MAO catalyst. These results clearly indicate that the present catalyst (4 – borate catalyst) should be effective for synthesis of ultrahigh molecular weight polymers and would suggest a possibility of (quasi) living polymerisation (control of repeat units and synthesis of block copolymers) in this catalysis under certain optimised conditions.| Run | α-Olefin | Time | Yield/mg | TONb | Activityc | Mnd × 10−6 | Mw/Mnd |
|---|---|---|---|---|---|---|---|
| a Conditions: olefin 5.0 mL, cat. 0.25 μmol, −30 °C, Ti/[Ph3C][B(C6F5)4]/AliBu3 = 1.0/3.0/500.b TON (turnover number) = (mmol of α-olefin reacted)/(mmol-Ti).c Activity = kg-polymer/mol-Ti h.d GPC data in THF vs. polystyrene standards. | |||||||
| 22 | OC | 3 | 70.2 | 2500 | 5620 | 1.18 | 1.54 |
| 23 | OC | 5 | 125.4 | 4470 | 6020 | 1.59 | 1.65 |
| 24 | OC | 10 | 160.3 | 5710 | 3850 | 2.00 | 1.89 |
| 25 | OC | 20 | 198.7 | 7080 | 2380 | 1.97 | 2.04 |
| 26 | OC | 30 | 237.7 | 8470 | 1900 | 2.45 | 1.90 |
| 27 | DD | 15 | 110.3 | 2620 | 1770 | 0.95 | 1.73 |
| 28 | DD | 15 | 111.7 | 2660 | 1790 | 1.07 | 1.62 |
| 29 | DD | 20 | 157.0 | 3730 | 1880 | 1.32 | 1.99 |
| 30 | DD | 20 | 145.0 | 3450 | 1740 | 1.45 | 1.84 |
We have shown that synthesis of ultrahigh molecular weight polymers by polymerisation of higher α-olefin have been achieved simply by 4 – borate catalyst. The fact would also introduce a possibility for synthesis of a new class of poly(α-olefin)s that possess highly branched (brush, cylindrical etc.) structure; further application by introduction of functionality into the side chain (for additional functionality, modification) would be thus highly expected.20,21 We are now exploring a possibility of not only (quasi) living polymerisation under certain optimised conditions, but also for synthesis of block copolymers and/or star polymers based on polyolefin main chain by adopting a certain living polymerisation technique. We believe that these should introduce a new possibility as promising materials based on poly(α-olefin)s.
Experimental section
General procedure
All experiments were carried out under a nitrogen atmosphere in a Vacuum Atmospheres drybox unless otherwise specified. All chemicals used were of reagent grade and were purified by the standard purification procedures. Anhydrous grade of toluene (Kanto Kagaku Co. Ltd) was transferred into a bottle containing molecular sieves (mixture of 3A and 4A 1/16, and 13X) in the drybox, and was used without further purification. Reagent grade 1-octene (TCI Co., Ltd.), 1-decene (TCI Co., Ltd.), 1-dodecene (Kanto Chemical Co., Inc.), 1-hexadecene (Mitsubishi Chemical Co.) and 1-octadecene (Mitsubishi Chemical Co.) were stored in bottles in the drybox and were passed through an alumina short column prior to use. Toluene and AlMe3 from commercially available methylaluminoxane [TMAO, 9.5 wt% (Al) toluene solution, Tosoh Finechem Co.] were removed under reduced pressure (at ca. 50 °C for removing toluene and AlMe3 and then heated at >100 °C for 1 h for completion) in the drybox to give white solids. Cp*TiCl2(O-2,6-iPr2C6H3) (1)22 and Cp*TiMe2(O-2,6-iPr2C6H3) (4)16 were prepared according to the reported procedure. [Me2Si(C5Me4)(NtBu)]TiCl2 (2, MCAT GmbH) and Cp2ZrCl2 (3, Aldrich) were used as received.All 1H and 13C NMR spectra were recorded on a Bruker AV 500 spectrometer (500.13 MHz for 1H; 125.77 MHz for 13C), and all chemical shifts are given in ppm and are referred to SiMe4. 13C NMR spectra for the resultant polymers were recorded with proton decoupling, and the pulse interval was 5.2 s, the acquisition time was 0.8 s, the pulse angle was 90°, and the number of transients accumulated was about 6000. The polymer samples for analysis were prepared by dissolving the polymers in CDCl3 solution, and the spectra was measured at 25 °C.
Molecular weights and the molecular weight distributions of the resultant polymers were measured by gel-permeation chromatography (GPC). HPLC grade THF was used for GPC and was degassed prior to use. GPC was performed at 40 °C on a Shimadzu SCL-10A using a RID-10A detector (Shimadzu Co. Ltd.) in THF (containing 0.03 wt% of 2,6-di-tert-butyl-p-cresol, flow rate 1.0 mL min−1). GPC columns (ShimPAC GPC-806, 804 and 802, 30 cm × 8.0 mm diameter, spherical porous gel made of styrene/divinylbenzene copolymer, ranging from <102 to 2 × 107 MW) were calibrated versus polystyrene standard samples. The molecular weight was calculated by a standard procedure based on the calibration with standard polystyrene samples.
Differential scanning calorimetric (DSC) data for the polymer were recorded by means of Hitachi DSC-7020 instrument under a nitrogen atmosphere [preheating: from 30 to 250 °C (20 °C min−1), cooling to −100 °C under N2, and measurement from −100 to 250 °C (10 °C min−1) under N2]. This heating and cooling was repeated two times. Tm values were determined from the middle point of the phase transition of the second heating scan.
Polymerisation of higher α-olefins
α-Olefin (1-octene, 1-decene, 1-dodecene, 1-hexadecene or 1-octadecene) polymerisations were conducted in a 10 mL scale vial in the drybox. A prescribed amount of MAO white solid, prepared by removing toluene and AlMe3 from commercially available MAO (TMAO-S, Tosoh Finechem Co.) and α-olefin were charged into the vial in the drybox. After an addition of a toluene solution (1.0 mL) containing a prescribed amount of complex via a syringe, the reaction mixture was stirred magnetically for 30 min. After the above procedure, the mixture was then poured into iPrOH (150 mL) containing HCl (10 mL). The resultant polymer was collected and was adequately washed with iPrOH and then dried in vacuo.The polymerisations in the presence of AliBu3 and [Ph3C][B(C6F5)4] were performed as follows: α-olefin (5.0 mL) and prescribed amount of AliBu3 were added into a 25 mL scale Schlenk flask under N2 at −30 °C. A toluene solution containing a prescribed amount of complex [at −30 °C] was added into the above solution, and the polymerisation was then started by addition of borate compound dissolved in toluene (1.0 mL). The reaction mixture was stirred for prescribed time. After the above procedure, the mixture was then poured into iPrOH (150 mL) containing HCl (10 mL). The resultant polymer was collected and was adequately washed with iPrOH and then dried in vacuo.
Acknowledgements
The present research is partly supported by Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS, No. 15H03812). S. P. expresses her thanks to Tokyo metropolitan government (Asian Human Resources Fund) for pre-doctoral fellowship, and the project was partly supported by the advanced research program (Tokyo metropolitan government). The authors also express their thanks to Tosoh Finechem Co. for donating MAO (TMAO), and express their thanks to Profs. A. Inagaki, K. Tsutsumi, S. Komiya (TMU) for discussions.Notes and references
- Selected reviews/accounts in 1990's, see: (a) G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428 CrossRef CAS; (b) A. L. McKnight and R. M. Waymouth, Chem. Rev., 1998, 98, 2587 CrossRef CAS PubMed; (c) J. Suhm, J. Heinemann, C. Wörner, P. Müller, F. Stricker, J. Kressler, J. Okuda and R. Mülhaupt, Macromol. Symp., 1998, 129, 1 CrossRef CAS; (d) W. Kaminsky and M. Arndt, Adv. Polym. Sci., 1997, 127, 144 CrossRef; (e) M. Bochmann, J. Chem. Soc., Dalton Trans., 1996, 255 RSC; (f) T. J. Marks, Acc. Chem. Res., 1992, 25, 57 CrossRef CAS; (g) R. F. Jordan, Adv. Organomet. Chem., 1991, 32, 325 CrossRef CAS; (h) Metalorganic Catalysts for Synthesis and Polymerisation: Recent Results by Ziegler–Natta and Metallocene Investigations, ed. W. Kaminsky, Springer-Verlag, Berlin, 1999 Search PubMed.
- Selected reviews, accounts, books, see: (a) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS PubMed; (b) A. F. Mason and G. W. Coates, in Macromolecular Engineering, ed. K. Matyjaszewski, Y. Gnanou and L. Leibler, Wiley-VCH, Weinheim, Germany, 2007, vol. 1, p. 217 Search PubMed; (c) Metal Catalysts in Olefin Polymerisation, ed. Z. Guan, Topics in Organometallic Chemistry 26, Springer-Verlag, Berlin, 2009 Search PubMed; (d) K. Nomura and S. Zhang, Chem. Rev., 2011, 111, 2342 CrossRef CAS PubMed; (e) H. Makio, H. Terao, A. Iwashita and T. Fujita, Chem. Rev., 2011, 111, 2363 CrossRef CAS PubMed; (f) M. Delferro and T. J. Marks, Chem. Rev., 2011, 111, 2450 CrossRef CAS PubMed; (g) C. Redshaw and Y. Tang, Chem. Soc. Rev., 2012, 41, 4484 RSC; (h) Orgaometallic Reactions and Polymerisation, ed. K. Osakada, The Lecture Notes in Chemistry 85, Springer-Verlag, Berlin, 2014 Search PubMed; (i) J. P. McInnis, M. Delferro and T. J. Marks, Acc. Chem. Res., 2014, 47, 2545 CrossRef CAS PubMed . Metal–metal cooperative effects in olefin polymerisation.
- Selected special issues: (a) Frontiers in Metal-Catalyzed Polymerisation (special issue); J. A. Gladysz, Chem. Rev., 2000, 100, 1167 CrossRef CAS PubMed; (b) Metallocene complexes as catalysts for olefin polymerisation; H. G. Alt, Coord. Chem. Rev., 2006, 250, 1 CrossRef CAS; (c) Metal-catalysed Polymerisation; B. Milani and C. Claver, Dalton Trans., 2009, 41, 8769 Search PubMed; (d) Advances in Metal-Catalysed Polymerisation and Related Transformations; P. Mountford, Dalton Trans., 2013, 42, 8977 RSC.
- Reviewing articles, accounts for half-metallocenes, see: (a) K. Nomura, J. Liu, S. Padmanabhan and B. Kitiyanan, J. Mol. Catal. A: Chem., 2007, 267, 1 CrossRef CAS; (b) K. Nomura, Dalton Trans., 2009, 8811 RSC; (c) K. Nomura and J. Liu, Dalton Trans., 2011, 40, 7666 RSC.
- Selected reviewing articles for living polymerisation, see: (a) G. W. Coates, P. D. Hustad and S. Reinartz, Angew. Chem., Int. Ed., 2002, 41, 2236 CrossRef CAS; (b) G. J. Domski, J. M. Rose, G. W. Coates, A. D. Bolig and M. Brookhart, Prog. Polym. Sci., 2007, 32, 30 CrossRef CAS; (c) K. Rhett, Chem.–Eur. J., 2007, 13, 2764 CrossRef PubMed; (d) L. R. Sita, Angew. Chem., Int. Ed., 2009, 48, 2464 CrossRef CAS PubMed; (e) G. M. Miyake and E.-Y. X. Chen, Polym. Chem., 2011, 2, 2462 RSC; (f) A. Valente, A. Mortreux, M. Visseaux and P. Zinck, Chem. Rev., 2013, 113, 3836 CrossRef CAS PubMed . Coordinative chain transfer polymerisation (CCTP).
- Recent review article, (a) The Chemistry of Catalyst Activation: The case of Group 4 Polymerisation Catalyst. M. Bochmann, Organometallics, 2010, 29, 4711 CrossRef CAS. Related references are cited therein; (b) Discovery of Methylaluminoxane as Cocatalyst for Olefin Polymerisation. W. Kaminsky, Macromolecules, 2012, 4, 3289 CrossRef.
- For example, Presentation by S. Babik, Advances in Polyolefins 2015, L29, Santa Rosa, California, 2015 Search PubMed.
- J. Saito, Y. Suzuki and T. Fujita, Chem. Lett., 2003, 32, 236 CrossRef CAS.
- Examples for syntheses of ultrahigh molecular weight poly(1-butene), poly(1-hexene) using thiobis(phenoxy)titanium dichloride in the presence of water-modified MMAO cocatalyst, (a) M. Fujita and T. Miyatake, US 6,288,192 B1, 2001; (b) M. Fujita, Y. Seki and T. Miyatake, Macromol. Chem. Phys., 2004, 205, 884 CrossRef CAS; (c) M. Fujita, Y. Seki and T. Miyatake, J. Polym. Sci. PartA: Polym. Chem., 2004, 42, 1107 CrossRef CAS.
- Synthesis of ultrahigh molecular weight poly(1-hexene) by (C5HMe4)2HfCl2 – MAO catalyst under ultrahigh pressure (ex. 250 MPa), A. Fries, T. Mise, A. Matsumoto, H. Ohmori and Y. Wakatsuki, Chem. Commun., 1996, 783 RSC.
- (a) K. Nomura, T. Komatsu and Y. Imanishi, J. Mol. Catal. A: Chem., 2000, 152, 249 CrossRef CAS; (b) K. Nomura, T. Komatsu and Y. Imanishi, J. Mol. Catal. A: Chem., 2000, 159, 127 CrossRef CAS.
- K. Nomura, K. Fujita and M. Fujiki, Catal. Commun., 2004, 5, 413 CrossRef CAS.
- (a) K. Nomura, T. Komatsu, M. Nakamura and Y. Imanishi, J. Mol. Catal. A: Chem., 2000, 164, 131 CrossRef CAS; (b) K. Nomura and A. Fudo, J. Mol. Catal. A: Chem., 2004, 209, 9 CrossRef CAS.
- For examples, see: (a) K. C. Jayaratne and L. R. Sita, J. Am. Chem. Soc., 2000, 122, 958 CrossRef CAS; (b) E. T. Kiesewetter and R. M. Waymouth, Macromolecules, 2013, 46, 2569 CrossRef CAS; (c) N. Nakata, T. Toda, T. Matsuo and A. Ishii, Macromolecules, 2013, 46, 6758 CrossRef CAS; (d) G. J. Domski, E. B. Lobkovsky and G. W. Coates, Macromolecules, 2007, 40, 3510 CrossRef CAS.
- These results were partly presented at Advances in Polyolefins 2015, Santa Rosa, California, September, 2015; Asian Polyolefin Workshop 2015 (APO2015), Tokyo, November, 2015; Pacifichem 2015, Honolulu, Hawaii, December, 2015.
- Data concerning effect of Al/Ti molar ratios in polymerisation of 1-decene, 1-dodecene using Cp*TiCl2(O-2,6-iPr2C6H3) (1) – MAO catalyst are shown in the ESI.†.
- MAO white solids by removing toluene and AlMe3 from commercially available MAO (methylaluminoxane; PMAO-S, Tosoh Finechem Co.) have been chosen as Al cocatalyst, because use of this MAO was effective in terms of catalytic activity and synthesis of high molecular weight polymers. K. Nomura, N. Naga, M. Miki and K. Yanagi, Macromolecules, 1998, 31, 7588 CrossRef CAS.
- Typical 1H and 13C NMR spectra in the resultant polymers and their DSC thermograms are shown in the ESI.†.
- Assignment for resonances in 13C NMR spectra for poly(α-olefin)s, see: T. Asakura, M. Demura and Y. Nishiyama, Macromolecules, 1994, 24, 2334 CrossRef.
- For examples (introduction of reactive functionality in polyolefin), see: (a) T. C. Chung, Functionalization of Polyolefins, Academic Press, San Diego, 2002 Search PubMed; (b) T. C. Chung, Prog. Polym. Sci., 2002, 27, 39 CrossRef CAS; (c) K. Nomura and B. Kitiyanan, Curr. Org. Synth., 2008, 5, 217 CrossRef CAS; (d) K. Nomura, J. Synth. Org. Chem., Jpn., 2010, 68, 1150 CrossRef CAS; (e) T. C. Chung, Green Sustainable Chem., 2012, 2, 29 CrossRef CAS; (f) W. Apisuk, K. Tsutsumi, H.-J. Kim, D.-H. Kim and K. Nomura, Green Sustainable Chem., 2014, 4, 133 CrossRef CAS.
- Example for an introduction of terminal olefinic double bonds by copolymerisation with 1,7-octadiene, (a) K. Nomura, J. Liu, M. Fujiki and A. Takemoto, J. Am. Chem. Soc., 2007, 129, 14170 CrossRef CAS PubMed; (b) W. Apisuk and K. Nomura, Macromol. Chem. Phys., 2014, 215, 1785 CrossRef CAS; (c) W. Zhao and K. Nomura, Macromolecules, 2016, 49, 59 CrossRef CAS.
- K. Nomura, N. Naga, M. Miki, K. Yanagi and A. Imai, Organometallics, 1998, 17, 2152 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Data concerning effect of Al/Ti molar ratios in polymerisation of 1-decene, 1-dodecene using Cp*TiCl2(O-2,6-iPr2C6H3) (1) – MAO catalyst, selected NMR spectra and DSC thermograms for polymers. See DOI: 10.1039/c5ra27797c |
| This journal is © The Royal Society of Chemistry 2016 |