Effect of hydrogen ion implantation on cholesterol sensing using enzyme-free LAPONITE®-montmorillonite electrodes
Nidhi Joshia,
Abhimanyu Sharmabc,
K. Asokanc,
Kamla Rawat*bc and
D. Kanjilalc
aSchool of Physical Sciences, Jawaharlal Nehru University, New Delhi 110067, India
bSpecial Center for Nanosciences, Jawaharlal Nehru University, New Delhi 110067, India. E-mail: kamla.jnu@gmail.com; Fax: +91 11 2674 1837; Tel: +91 11 2670 4699
cInter University Accelerator Centre (IUAC), New Delhi 110067, India
First published on 15th February 2016
Abstract
Ion irradiation experiments were performed on LAPONITE®-montmorillonite/indium tin oxide (L-MMT/ITO) films using a 20 keV H2+ ion beam with variable fluence ranging from 1012 to 1016 ions per cm2, and the electrochemical profiling of these irradiated electrodes was done using Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS). Scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) were used for pre and post-irradiation morphology study and binding analysis. FTIR spectroscopy indicated the implantation of low-energy H2+ ions resulting in the formation of new bonds. The enhanced cholesterol sensitivity of irradiated films up to a fluence of 1013 ions per cm2 was observed due to morphological changes taking place in L-MMT films. Close to 20% enhancement in cholesterol sensitivity was noticed, when the ion fluence was ≈1013 ions per cm2. The sensitivity for cholesterol detection of the L-MMT electrode formed through H2+ ion implantation clearly exhibited a strong dependence on the fluence of the ion beam. The radiation-induced enhanced sensitivity can be proposed as a platform for development of a more effective enzyme-free strip sensor.
I. Introduction
Ion beam irradiation has now become one of the interesting but routine techniques for processing as well as surface modification of systems comprising bulk material, thin films and nanostructures. Ion beam irradiation is known to be a technique used in various research fields including bioengineering, radioactive breeding, tumour therapy etc.1–6 The use of radiation in polymer surface/thin films, where the ion beam interacts with the material and results in the modification of their physico-chemical properties, such as optical absorption, electrical conductivity, thermal stability etc. is increasing rapidly.7–9Ion irradiation may result in severe damage, or enhancement of stability and/or conductivity of polymer thin films depending on the mass, energy and density of the interacting ions. Thus, its use can be tailored (used as tuning agent) to suit material processing. Ghisleni et al.10 have studied the effect of ion irradiation on TEOS (tetraethylorthosilicate) Si(OC2H5)4/MTES (methyltriethoxysilane) CH3Si(OC2H5)3 thin film resulting in the structural transformation of the films which led to increase in elastic modulus, and hardness of the film. A study of surface modification of poly(lactic-co-glycolic) acid due to irradiation was done using argon ion irradiation by Adhikari et al.11 and structural changes were confirmed using X-ray photoelectron and atomic force spectroscopy. Ion bombardment promotes the formation of unsaturated bonds or the breakage of polymeric chains or emission of fragments that may be atomic or/and molecular.12
The studies of nano-biosensors for the determination of concentration of analytes (glucose, cholesterol, ascorbic acid and oxalic acid etc.) which are of clinical interest have received considerable attention recently.13 Cholesterol is known to be one of the main metabolites of human body being an active component of nerve and brain cells.14 Cholesterol is one of the analytes which is determined in clinical pathology with its concentration being indeed indicatively related to human health. The determination of cholesterol is important for diagnosis and prevention of a number of clinical disorders like hypertension, coronary heart disease etc. Thus, it is of interest to both the biological science and food industries. The content of cholesterol in normal human blood serum is less than 200 mg dl−1 of which 70% is esterified with fatty acids and 30% is present as sterol.15 In literature, there have been a lot of attempts to develop cholesterol biosensors using enzyme cholesterol oxidase as well as without enzymes which is listed in Table 2.
| Fluence (ions per cm2) | 2θ (°) | FWHM (°) | Crystallite size (Å) |
|---|---|---|---|
| 0 | 7.46 | 0.0156 | 92.30 |
| 1 × 1012 | 7.37 | 0.0189 | 76.44 |
| 1 × 1014 | 7.36 | 0.0206 | 70.09 |
| S. no. | Electrode | Enzyme used | Linear dynamic range | Sensitivity | Limit of detection | References |
|---|---|---|---|---|---|---|
| 1 | SiO2/CHIT/MWCNT/ITO | ChEt/ChOx | 10–500 mg dl−1 | 3.84 μA mM−1 | — | Solanki et al., 2009 (ref. 31) |
| 2 | (p(HEMA))/(polypyrrole) | ChOx | 5 × 10−4 to 1.5 × 10−2 M | 6.63 nA mM−1 | 120 μM | Brahim et al., 2001 (ref. 32) |
| 3 | Polypyrrole (PPY) | ChEt/ChOx | 1–8 mM | 0.15 μA mM−1 | — | Singh et al., 2004 (ref. 33) |
| 4 | Ag nano-particle/GCE | None | 2.8 × 10−4 to 3.3 × 10−2 M | — | 1.8 × 10−4 M | Li et al., 2010 (ref. 34) |
| 5 | CeO2-graphene | None | 12 μM to 7.2 mM | — | 4 μM | Zhang et al., 2013 (ref. 35) |
| 6 | PtNP/(CNT) | None | 0.005–10 mM | 8.7 μA mM−1 cm−2 | — | Yang et al., 2012 (ref. 36) |
| 7 | CSNF-AUNPs | ChOx | 1–45 μM | 1.02 μA mM−1 | — | Gomathi et al., 2011 (ref. 37) |
| 8 | ZnO nanoparticle | ChOx | 1–500 nM | 2.3 μA mM−1 cm−2 | 0.37 nM | Umar et al., 2009 (ref. 38) |
| 9 | Irradiated L-MMT | None | 1–20 mM | 8.08 μA mM−1 cm−2 | 1 mM | Present work |
Nanoclays such as Na montmorillonite (Cloisite Na) MMT are materials naturally occurring in the clay fraction of soil. In contrast, LAPONITE® RD is synthetic clay developed by Laporte Industries. These clays have found wide agricultural, industrial and mechanical applications. Thus, LAPONITE® with a face diameter of ∼30 nm and MMT with diameter of ∼300 nm, and rim width of 1 nm each are known to be rheology modifier and have various applications in agriculture, building materials, surface coatings, household and personal care items.16,17 Nanoclay has the advantageous properties of high chemical stability, good absorption and penetrability due to its large surface area, and thus, its use in sensing can be promising. M. Seleci et al.18 have developed a clay-based biosensor where they used MMT modified with methyl and dimethylamine to analyse glucose in wine.
In the present work, the objective was to study the influence of low energy (20 keV) H2+ ion beam of different fluence on L-MMT/ITO electrode resulting in the alteration in the properties (both morphological and electrochemical) of electrodes for detection of cholesterol. The effect of ion induced modification in the sensitivity of the thin films was also explored. We have shown that the variation in fluence of molecular hydrogen ions can provide conditioning of the sensitivity of nanoclay-based electrodes towards the enhanced sensing of cholesterol at room temperature on an enzyme-free platform.
II. Materials and method
LAPONITE® RD and Cloisite-Na (MMT) were purchased through Southern Clay Products, U.S. The individual dispersion of LAPONITE® and MMT were prepared by dissolving 1.25% (w/v) in deionized water for 2 h and 12 h, respectively. After their preparation, both the dispersions were mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and stirred vigorously for half an hour. As the homogeneous dispersions were prepared, 40 μl of the dispersion were drop casted onto ITO electrode and kept for drying for ≈2 days. After the preparation of electrode, these were irradiated with 20 keV hydrogen molecular ion (H2+) beam having different fluences (1012 to 1016 ions per cm2), and then used for further analysis. The pH of the prepared L-MMT solution was found to be 9.0 ± 0.5.
1 ratio and stirred vigorously for half an hour. As the homogeneous dispersions were prepared, 40 μl of the dispersion were drop casted onto ITO electrode and kept for drying for ≈2 days. After the preparation of electrode, these were irradiated with 20 keV hydrogen molecular ion (H2+) beam having different fluences (1012 to 1016 ions per cm2), and then used for further analysis. The pH of the prepared L-MMT solution was found to be 9.0 ± 0.5.
The electrochemical studies (cyclic voltammetry and electrochemical impedance spectroscopy) were done on Autolab Potentiostat/Galvanostat (Eco Chemie, Netherlands) in a three-electrode cell dipped in 3.3 mM ferric and ferrous solution containing KCl. The three-electrode cell consisted of working electrode that was L-MMT/ITO, auxiliary electrode which was a platinum wire, and reference electrode that was Ag/AgCl. Fourier transform infrared (FTIR) spectroscopic studies were done on PerkinElmer, Spectrum BX II instrument. X-Ray diffraction (XRD) measurements were carried out on XRD, RigakuD/Max 2200 diffractometer with Cu-Kα radiation with wavelength of 1.54 Å in the 2θ range of 3–30°. Scanning electron microscope (SEM) imaging was carried out using SEM, Zeiss, EVO-40 instrument. Ion beam irradiations on L-MMT samples were done using Table Top 50 kV Ion accelerator which is one of the low energy ion beam facility (LEIBF) available at IUAC.19
III. Results and discussion
(a) Electrochemical response
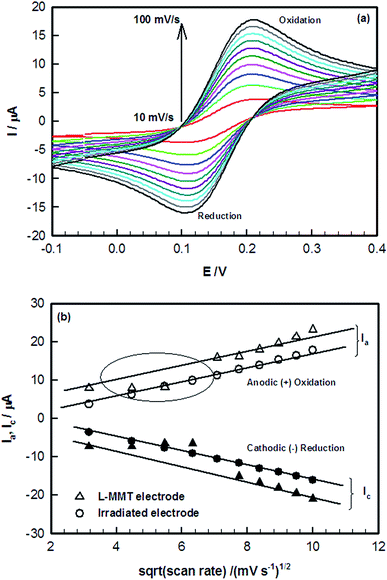
Cyclic Voltammetry (CV) is known to be a common technique which provides information about redox potentials of the sample. The cyclic voltammetry studies of the electrodes before and after irradiation with hydrogen molecular ion (H2+) beam using 1012, 5 × 1012, 1013, 5 × 1013, 1014, 5 × 1014, 1015, 5 × 1015 and 1016 ions per cm2 fluences were carried out using Zobell's solution {3.3 mM K4Fe(CN)6 (potassium ferrocyanide), 3.3 mM K3Fe(CN)6 (potassium ferricyanide) containing 0.1 M KCl (potassium chloride)} at pH 7. The cyclic voltammogram were recorded in the potential range of −0.1 to 0.4 V. The various tuning parameters such as scan rate, pH, concentration etc. were optimized in an earlier work.20 Fig. 1(a) shows the cyclic voltammogram of L-MMT electrode with, and without irradiation (1014 ion per cm2) at different scan rates varying from 10 to 100 mV s−1. The electrochemical profiles of all the L-MMT thin films (with and without irradiation) showed qualitatively same behaviour with the oxidation and reduction process occurring at same potential of 0.22 V and 0.12 V. Fig. 1(b) shows the scan rate dependence of peak current where both the anodic and cathodic current showed increasing behaviour with square root of scan rate. Thus, for both with and without irradiated samples, the anodic (Ia) and cathodic (Ic) current increased linearly with square root of scan rate with regression coefficient value (R2) of 0.98 and 0.96 for irradiated (1014 ions per cm2) and non-irradiated electrodes indicating that the electron transfer process was a diffusion controlled process.21 The same result was found for all the other fluences.Further electrochemical studies on all the samples were done in ferri/ferro solution at pH 7, and at the scan rate of 50 mV s−1. The effect of irradiation is shown in Fig. 2 where the electrochemical response of electrode irradiated with variant fluences was compared. For low fluence H2+ ion beam (≤1013 ion per cm2), the increase in magnitude of anodic and cathodic peak current were observed compared to L-MMT electrode. However, the decrease in anodic peak current for high fluence H2+ ion beam (>1013 ions per cm2) compared to L-MMT/ITO electrode was clearly noticed. The hydrogen molecular (H2+) ion beam irradiation to L-MMT/ITO electrode resulted in the incorporation of these ions from the beam and thus, resulted in enhancement of the anodic and cathodic currents. Both the current showed increasing behaviour upto 1013 ions per cm2 fluences while for higher fluences, it showed decreasing trend. This figure also shows that in the absence of cholesterol the fluence of hydrogen molecular ion beam had very negligible effect on the current value while noticeable changes were observed in the presence of cholesterol. Thus, the irradiated electrode, with low fluence, provided favourable environment for the electron transfer process in the electrode/electrolyte interface for the cholesterol, but at higher fluence, the samples provided hindrance to the electron transfer process due to excessive damage to the electrode surface.
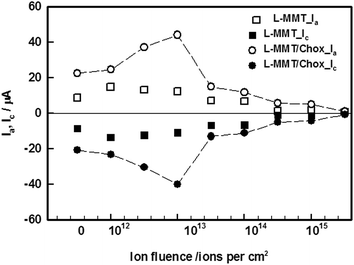 | ||
| Fig. 2 Anodic and cathodic peak current as a function of ion fluences of H2+ ion beam in the absence and presence of cholesterol. | ||
At low fluence of irradiation, sufficient accessibility to electrons between electrolyte and electrodes is facilitated. Further, we proceed to calculate diffusion coefficient value from ferri/ferro electrolyte to different electrodes using the Randles–Sevcik22 equation given by
| Ip = (2.69 × 105)n3/2AD1/2CV1/2 | (1) |
The electro-active surface area of electrode was determined from the calculated diffusion coefficient (D) and the Randles–Sevcik22 equation given by
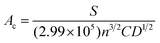 | (2) |
Also, the value of the electron transfer rate constant (Ks) for different electrodes were determined using eqn (3) which was based on model of Laviron23 given by
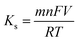 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1 and R is the universal gas constant.
485 C mol−1 and R is the universal gas constant.
The surface concentration (I*) of ionic species of the corresponding electrode were calculated through Brown–Anson model22
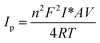 | (4) |
The diffusion coefficient value of redox species of bare (not irradiated) L-MMT electrode was found to be 0.306 × 10−12 cm2 s−1 while irradiated L-MMT electrode has diffusion coefficient as 0.890 × 10−12 cm2 s−1. Therefore, irradiation led to increase in electron diffusion coefficient of the electrode. The charge transfer rate constant and average surface concentration for non-irradiated L-MMT electrode was found to be 0.194 s−1 and 0.07 × 10−8 mol cm−2.
An increase in anodic current was observed with cholesterol concentration in the range of 1–20 mM (Fig. 3). The magnitude of the oxidation peak (Ia) increased due to the increasing concentration of cholesterol. The maximum increase in magnitude of anodic and cathodic peak current with cholesterol was found for lower fluences of ion beam which revealed faster electron transfer process between electrode and electrolyte.
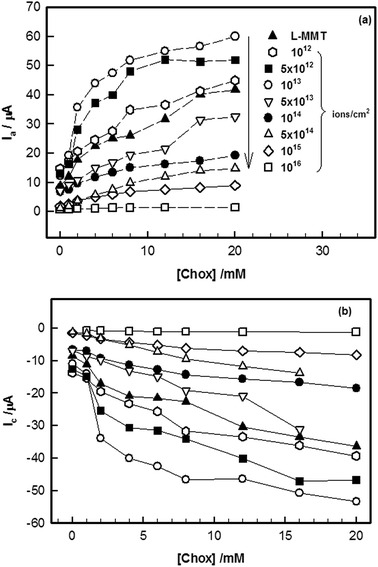 | ||
| Fig. 3 Variation of (a) anodic (Ia) and (b) cathodic (Ic) peak current with the concentration of cholesterol in the range of 1–20 mM shown for different ion beam fluence. | ||
Increase in current, with increase in cholesterol concentration, showed the presence of interaction of cholesterol with L-MMT electrode.24 Thus, addition of cholesterol in the electrolyte provided an easy conducting path for the electron to transfer from electrode to electrolyte or vice versa for low fluence irradiated electrode.
The sensitivity which is defined as output of the sensor per unit input, of the L-MMT electrode toward cholesterol was calculated from the slope of the current versus cholesterol concentration plot. The sensitivity for electrode irradiated with different fluence is shown in Fig. 4 where a clear enhancement of sensitivity for electrode which was irradiated with low fluence (≤1013 ions per cm2) was noticed. In addition to this, loss of sensitivity was found for higher fluence (>1013 ions per cm2) irradiated electrodes.
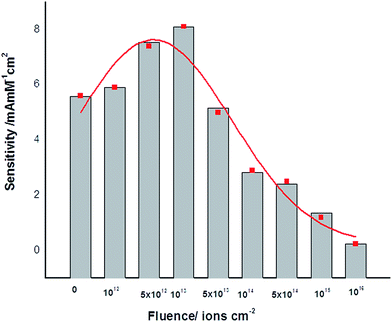 | ||
| Fig. 4 Sensitivity of L-MMT/ITO electrode for different irradiated electrode was shown, where the maximum sensitivity was noted for the H2+ ion beam at the fluence of 1013 ions per cm2. | ||
A well-known phenomenon which was observed in ion irradiation for various Si based polymer thin films is the loss of hydrogen which was due to breakage of hydrogen bonds.25–28 F. Z. Cui et al.25 studied the changes in protein secondary structure by low energy nitrogen ion beam irradiation due to the scission of H-bonds and charge exchange. Polymer films synthesized from plasmas of a tetra methylsilane–Ar mixture using helium ion beam irradiation showed loss of hydrogen was reported by R. V. Gelamo et al.26 E. C. Rangel et al.27 investigate the effect of argon ion irradiation on polymer film where significant loss of hydrogen besides the formation of unsaturated bonds, and cross-links with decrease in electrical resistivity was found. Also the loss of hydrocarbon and the associated release of hydrogen is one of the consequences of the ion irradiation of polymer and polymer-like structures studied by D. L. Baptista et al.28 Here, such a mechanism is hypothesized for the L-MMT colloidal thin film that the enhancement in sensitivity of the irradiated electrode could be due to the breakage of hydrogen bond induced by the as deposited L-MMT/ITO electrode. These liberated hydrogen atoms can diffuse out of the matrix resulting in the formation of active sites or defects which may result in increase in electron transfer (redox) process. The high fluence of ion beam result in the recombination of these liberated hydrogen atoms, and may block the active sites which were earlier responsible for the electron transfer kinetics in low fluence irradiated electrode. Thus, high fluence provided the hindrance (blockage) to the transfer process, and resulted in reduction of the sensitivity of the L-MMT electrode towards cholesterol due to physical degradation or damage to the current flow. The observation of enhancement of sensitivity by irradiation of hydrogen (H2+) molecular ion to L-MMT electrode clearly exhibited dependence on the fluence. From the CV analysis, low fluence irradiated electrode showed enhancement in the electron transfer process, and thus, helped in increasing the sensitivity towards this specific analyte (cholesterol). This is clearly depicted in Fig. 4.
(b) Electrochemical Impedance Spectroscopy (EIS)
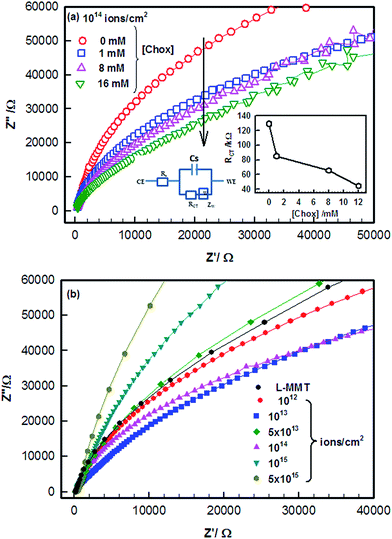
Now, Electrochemical Impedance spectroscopy (EIS) study was done on the electrode in the frequency range of 0.01 to 106 Hz so as to probe the surface of modified electrode. The Nyquist plot for electrochemical impedance (Z′′ vs. Z′) of L-MMT electrode is shown in the Fig. 5 and it provided information about the material conductivity, and dielectric constant.The impedance data were analysed using Randle's equivalent circuit results from the parallel combination of electron transfer resistance (RCT) and double layer capacitance (Cdl) from electrode impedance. The semicircle in the Nyquist plot corresponds to the electron transfer limited process. The electron transfer resistance (RCT) value was calculated from the diameter of the semicircle which defined the electron transfer process of the redox probe. The decrease in diameter of semicircle with the addition of cholesterol showed the low resistivity value and thus the addition of cholesterol enhanced the electron transfer process (conducting) and provided better environment for cholesterol to interact with L-MMT (Fig. 5(a)).
A depressed semicircle for irradiated L-MMT in the presence of cholesterol having small diameter reflects the faster electron transfer process from electrode to electrolyte and vice versa as compared to the large semicircle which reflects the greater hindrance to the transfer process. The diameter of the semicircle of impedance spectra in Fig. 5(b) provides the value of electron transfer resistance (RCT) of the electrode surface which varies from 319 KΩ to 38 KΩ for low fluences (≤1013 ions per cm2) while increases from 38 KΩ to 426 KΩ for high fluences (>1013 ions per cm2). This decrease in RCT value shows the enhanced electron transfer pathway from electrode to electrolyte and vice versa. The ion beam irradiation of L-MMT/ITO electrode led to dielectric behaviour for electron transfer process. The high fluence ion beam irradiation resulted in the creation of a barrier (blockage due to recombination) so that electron transfer process couldn't occur easily as observed through the Nyquist plot (Fig. 5(b)). The decrease in RCT value upon addition of cholesterol to the electrolyte solution can be related to surface effects and it's binding with the irradiated electrode. Thus, irradiation with low fluence results in improved charge transfer due to breakage of hydrogen bonds resulting in formation of active sites which enhanced diffusion of ferri/ferrocyanide ions towards the electrode surface and vice versa. Hence, consistent results were obtained when the electrode was studied using electrochemical techniques (cyclic voltammetry and electrochemical impedance spectroscopy).
(c) Surface morphology
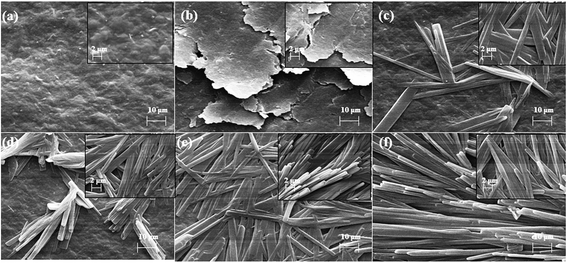
Ion irradiation can lead to the alteration in the surface morphology, which in turn may contribute to the changes in the electrochemical properties of the L-MMT electrode. The surface morphology of irradiated L-MMT electrode was studied by Scanning electron microscope (SEM), which shows huge irregularly flakes of cholesterol distributed on the surface of irradiated L-MMT (Fig. 6(b)). These images show that with increase in ion fluence of ion beam, there is an increment of coverage area through flakes of cholesterol on the surface. For low fluence (≤1013 ions per cm2) the ion implantation result in breakage of hydrogen bond and formed the sites which were responsible for interaction of cholesterol. While for higher ion fluence (>1013 ions per cm2) the further recombination of hydrogen atoms takes place and thus blocked the sites for cholesterol. This result in hindrance for cholesterol to interact with high fluence irradiated L-MMT electrode and thus self-assembled irregular flakes of cholesterol were found on the whole surface. The images of irradiated electrode for high fluence in the presence of cholesterol showed rod like structures (huge irregular flakes) which were formed due to the linear self-assembly of the analyte (cholesterol). Therefore, the best change in surface morphology was found for low ion fluence which implied that incoming cholesterol was successfully aligned on the surface of pre-irradiated L-MMT electrode.It was concluded that irradiation of the L-MMT electrode results in the alteration in surface morphology as observed from the SEM images and this provided the enhanced sensitivity for detection of cholesterol in case of low fluence of ion beam.
(d) X-ray diffraction
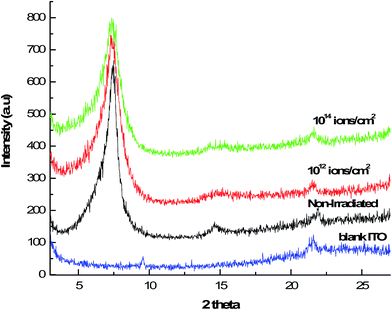
X-ray diffraction techniques (XRD) were used to investigate structural properties. Fig. 7 shows the XRD patterns of the samples without and with irradiation of 20 KeV H2+ ion beam at the fluence of 1012 ions per cm2 and 1014 ions per cm2. It was found that the broadness of the irradiated electrodes increases compared to non-irradiated one due to the destruction of crystallinity. The size of the crystallite (L) can be obtained from Scherrer's29 equation given as
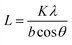 | (5) |
The crystallite size was found to decrease with increase in ion fluence of H2+ irradiated beam. Thus, irradiation of L-MMT thin film with hydrogen molecular ion beam causes 20% and 31% of decrease in crystallite size for low and high fluence of 1012 ions per cm2 and 1014 ions per cm2. XRD measurements thus provide the information of loss of crystallinity due to irradiation.
(e) FTIR
FTIR spectroscopy studies were used to quantify the chemical and structural transformation induced by ion irradiation. The FTIR spectrum for irradiated L-MMT thin films in the spectral range of 500–4000 cm−1 is shown in Fig. 8(a) and (b). The bombardment of hydrogen molecular (H2+) ion beam on the L-MMT electrode may result in the alteration of their structure.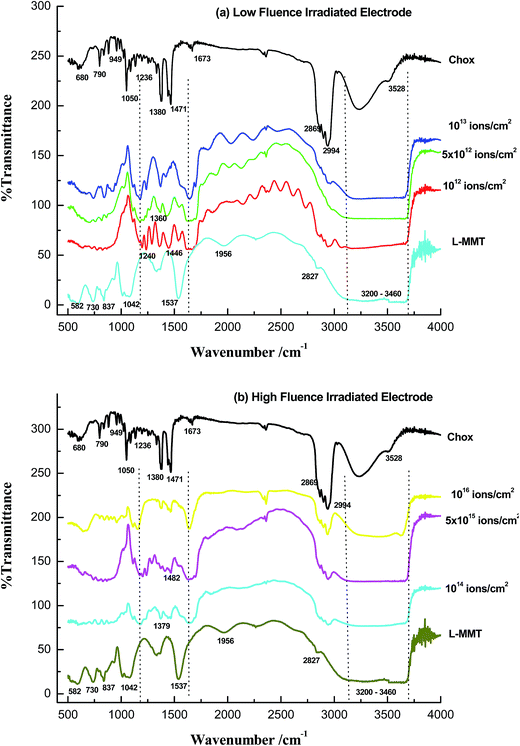 | ||
| Fig. 8 FTIR spectroscopic data for L-MMT/cholesterol/ITO irradiated with (a) low fluence and (b) high fluence of ion beam. | ||
The cholesterol consists of C–H bond, O–H bond, C–O bond, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, and C–C bond. The obtained FTIR spectra for pure cholesterol shows peak at 680 and 790, 1050 and 1236, 1380, 1471, and, 859, 949 and 1037 cm−1 corresponding to the bonds C–H, O–H (of hydroxyl group), C–O (between carbon and hydroxyl), C
C bond, and C–C bond. The obtained FTIR spectra for pure cholesterol shows peak at 680 and 790, 1050 and 1236, 1380, 1471, and, 859, 949 and 1037 cm−1 corresponding to the bonds C–H, O–H (of hydroxyl group), C–O (between carbon and hydroxyl), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (unsaturated carbon carbon) and C–C respectively.30 Besides this peak at 2869 and 2994 cm−1 corresponds to C–H bonds having asymmetric mode of stretching.
C (unsaturated carbon carbon) and C–C respectively.30 Besides this peak at 2869 and 2994 cm−1 corresponds to C–H bonds having asymmetric mode of stretching.
For L-MMT nanoclay, the spectrum shows a broad band at 3200–3460 cm−1 attributed to OH stretching due to presence of water. The broad bending band at 1042 cm−1 and the stretching band at 837 cm−1 attributes to the presence of –Si–O–Si– known to be siloxane groups. The characteristic of an asymmetric stretching vibration of Si–O–Si was found from the occurrence of these peaks. The bands which are located at 582 cm−1 and 730 cm−1 was associated with the stretching vibration of Si–OH or Si–O− bonds. Bands at around 3200–3460 cm−1 and 1537 cm−1 were associated with the stretching and deformation vibrations of the interlayer H2O of nanoclay. The small band at 2827 cm−1 was due to the asymmetrical stretching vibration of C–H in CH2 and CH3 and this presence of alkyl chain in the surface of electrode was due to the cholesterol which was drop-cast over the L-MMT electrode for observing the interactions.
The effect of irradiation on the L-MMT thin films can be noted by observing the gradual disappearance or reduction or shifting of various vibration bands. As it can be seen in Fig. 8(a), after the L-MMT electrodes were irradiated with H2+ ion beam of low fluences the band around 1537 cm−1 was shifted to 1637 cm−1 which suggest that the irradiation result in increment in interlayer H2O deformation due to the breakage of hydrogen bond.
The broadness of the band at 1637 cm−1 was found to increase for low fluences due to the increase in interaction and bonding with the hydroxyl group of cholesterol while it decreased for higher fluences. In addition to this, there were occurrence of sharp peaks correspond to cholesterol (1240, 1360, 1446 cm−1) in Fig. 8(a) which occurs for low fluence in region 1200–1600 cm−1 confirming the interaction of L-MMT with the cholesterol due to irradiating ions which lead to breakage of hydrogen bonds and this enhanced the electrochemical properties of the electrode. However, high fluence results in disappearance (collapse) of these peaks due to recombination of hydrogen atom resulting in less interaction or sensing of electrode towards cholesterol detection (Fig. 8(b)). These peaks arises due to interaction of O–H bending and C–O stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations. The disappearance of various peaks in region 1600–2800 cm−1 shows the less binding (interaction) of cholesterol with L-MMT for high fluence as compared to the low fluence case. For high fluence, the diminished of peak 1240 cm−1 and shifting of 1360 cm−1 and 1445 cm−1 shows the different behaviour of irradiated electrode with cholesterol. Thus, these results in the peak variation support the better interaction of irradiated electrode of low fluence with cholesterol.
C vibrations. The disappearance of various peaks in region 1600–2800 cm−1 shows the less binding (interaction) of cholesterol with L-MMT for high fluence as compared to the low fluence case. For high fluence, the diminished of peak 1240 cm−1 and shifting of 1360 cm−1 and 1445 cm−1 shows the different behaviour of irradiated electrode with cholesterol. Thus, these results in the peak variation support the better interaction of irradiated electrode of low fluence with cholesterol.
The decrease in conductivity for irradiated samples (higher fluence) was due to loss of hydrogen due to recombination process or formation of (any) unsaturated bonds. For high fluence of ion beam the samples are sensitive to ion radiation and were physically damaged to certain extent resulting in the decrease in sensitivity for detection of cholesterol. Fig. 9 represents the graphic representation of irradiated L-MMT/ITO electrode for sensing cholesterol.
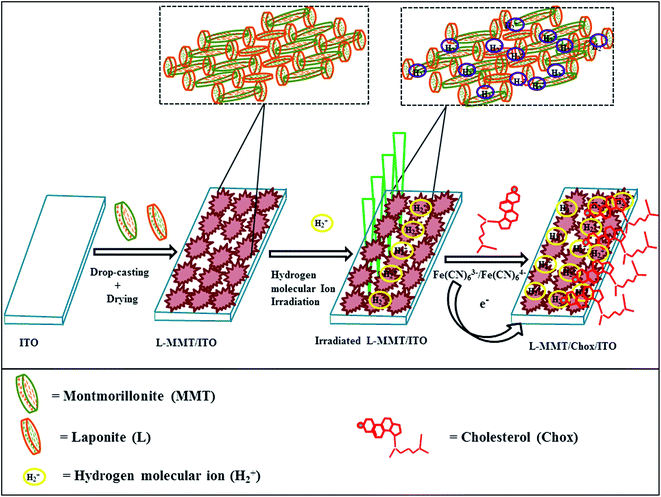 | ||
| Fig. 9 Schematic representation for fabrication method of L-MMT irradiated with H2+ ion beam for sensing cholesterol. | ||
IV. Conclusion
The hydrogen molecular ion (H2+) irradiation on the successfully fabricated L-MMT/ITO electrode was used for sensing of cholesterol. This work describes a follow-up study of our previous work where we found electrocatalytic behaviour of L-MMT/ITO electrode towards the detection of cholesterol.20 The sensing mechanism used for L-MMT toward cholesterol detection was based on ferro-ferri cyanide redox electrochemical reaction. Irradiation of ion beam produced surface modification of these electrodes and enhancement of sensitivity was noticed for low fluence (upto 1013 ions per cm2) towards cholesterol due to the breakage of hydrogen bonds. Irradiation by high fluence ions (above 1013 ions per cm2) caused the degradation of the electrode surface and resulted in offering lesser sensitivity due to the recombination of hydrogen atom. A mechanism is hypothesized for the electrode that low fluence irradiation induced certain active sites by breaking hydrogen bonds and these liberated hydrogen atoms were responsible for controlling (enhancing) the electron transfer kinetics. From this, we further concluded that the fluence provides the tunability of the implantation induced sensitivity of the L-MMT/ITO electrodes. In addition to this, low fluence irradiations from hydrogen molecular ion (H2+) beam on L-MMT electrode were found to be successful for enhancing electrochemical properties of the electrode. In general, such electrodes could be used for detection of cholesterol under room temperature conditions. This sensing platform can be developed to design cheap enzyme-free strip sensor for mass production and day to day use.Acknowledgements
This study was funded by the Department of Science and Technology (DST), India. NJ acknowledges CSIR, Government of India for Senior Research Fellowship. KR is thankful to Department of Science and Technology, Government of India-Inspire Faculty Award. We are thankful to the Advanced Research Instrumentation Facility (AIRF) of the University for allowing us access to the SEM and FTIR facility and Inter University Accelerator Centre (IUAC) for Hydrogen ion beam irradiation facility. We are also thankful to Mr Rajkumar (IUAC) for his support during ion beam irradiation. Helpful discussion with Prof. H. B. Bohidar of School of Physical Sciences, JNU, Dr P. R. Solanki of Special Center for Nanosciences, JNU and Dr G. B. S. Lakshmi of IUAC is acknowledged.References
- Z. L. Yu, Introduction to Ion Beam Biotechnology, ed. L. D. Yu, T. Vilaithong and I. Brown (trans.), Springer, New York, 2006 Search PubMed.
- S. R. Forrest, M. L. Kaplan, P. H. Schmidt, T. Venkatesan and A. J. Lovinger, Appl. Phys. Lett., 1982, 41, 708–712 CrossRef CAS.
- D. Schulz-Ertner, O. Jakel, W. Schlegel and R. Semin, Radiat. Oncol., 2006, 16, 249–259 CrossRef PubMed.
- Y. Fujinami, H. Hayashi, A. Ebe, O. Imai and K. Ogata, Mater. Chem. Phys., 1998, 54, 102–105 CrossRef CAS.
- A. L. Ruoff, E. J. Kramer and C. Y. Li, IBM J. Res. Dev., 1988, 32, 626–630 CrossRef CAS.
- P. Bodo and J. E. Sundgren, J. Appl. Phys., 1986, 60, 1161–1168 CrossRef.
- A. Islam, T. Yasin and I. U. Rehman, Radiat. Phys. Chem., 2014, 96, 115–119 CrossRef CAS.
- J. S. C. Loo, C. P. Ooi and F. Y. C. Boey, Biomaterials, 2005, 26, 1359–1367 CrossRef CAS PubMed.
- K. Yoshihisa, A. Yoshimura, Y. Shibamori, K. Fuchigami and N. Kubota, J. Solid Mech. Mate. Eng., 2012, 6, 654–659 CrossRef.
- D. A. Lucca, R. Ghisleni, M. Nastasi, L. Shao, Y. Q. Wang, J. Dong and A. Mehner, Nucl. Instrum. Methods Phys. Res., 2007, 257, 577–580 CrossRef CAS.
- A. R. Adhikari, B. P. Tilakaratne, D. Wijesundera and W. K. Chu, J. Surf. Eng. Mater. Adv. Technol., 2014, 4, 326–331 CAS.
- E. H. Lee, in Polyimides: Fundamental Aspects and Technological Applications, ed. K. Mittal and M. Ghosh, Marcel Dekker, New York, 1996, ch. 17 Search PubMed.
- A. White, P. Handler, E. L. R. Smith, L. Hill and I. R. Lehman, Principles of Biochemistry, McGraw Hill Book, New York, 6th edn, 1978 Search PubMed.
- N. B. Myant, Cholesterol Metabolism, LDL, and the LDL Receptor, Academic Press, Inc., San Diego, California, 1990 Search PubMed.
- G. Li, J. M. Liau, G. Q. Hu, N. Z. Ma and P. J. Wu, Biosens. Bioelectron., 2005, 20, 2140–2144 CrossRef CAS PubMed.
- R. J. Hunter, Foundations of Colloid Science, Clarendon, Oxford, 1989, vol. 1 Search PubMed.
- Laponite-Synthetic layered silicate- its chemistry, structure and relationship to natural clays, Laponite technical bulletin, 2003, L204/01g, http://www.laponite.com.
- M. Seleci, D. Ag, E. E. Yalcinkaya, D. O. Demirkol, C. Guler and S. Timur, RSC Adv., 2012, 2, 2112–2118 RSC.
- R. Kumar, R. Ahuja, C. P. Safvan and A. Roy, Proceeding of the Indian particle accelerator conference, 2013, pp. 1–3 Search PubMed.
- N. Joshi, K. Rawat, P. R. Solanki and H. B. Bohidar, Biochem. Eng. J., 2015, 102, 69–73 CrossRef CAS.
- G. A. Mabbott, J. Chem. Educ., 1983, 60, 697–702 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, New York, 1980 Search PubMed.
- E. Laviron, J. Electroanal. Chem., 1979, 100, 263–270 CrossRef CAS.
- S. Zong, Y. Cao and H. Ju, Electroanalysis, 2007, 19, 841–846 CrossRef CAS.
- F. Z. Cui, Y. B. Lin, D. M. Zhang and M. B. Tian, Radiat. Phys. Chem., 2001, 60, 35–38 CrossRef CAS.
- R. V. Gelamo, S. F. Durrant, B. C. Trasferetti, C. U. Davanzo, F. P. M. Rouxinol and M. A. B. de Moraes, Plasma Processes Polym., 2007, 4, 489–496 CrossRef CAS.
- E. C. Rangel, N. C. da Cruz, M. A. B. de Moraes and C. M. Lepienski, Surf. Coat. Technol., 2000, 127, 93–98 CrossRef CAS.
- D. L. Baptista and F. C. Zawislak, Diamond Relat. Mater., 2004, 13, 1791–1801 CrossRef CAS.
- P. Scherrer, Nachr. Ges. Wiss. Gott, 1918, 2, 98–104 Search PubMed.
- R. M. Silverstein, G. C. Bassler and T. C. Morrill, in Spectroscopic Identification of Organic Compounds, John Wiley and Sons, Inc., New York, 5th edn, 1991, pp. 91–164 Search PubMed.
- P. R. Solanki, A. Kaushik, A. A. Ansari, A. Tiwari and B. D. Malhotra, Biochem. Eng. J., 2015, 102, 69–73 CrossRef.
- S. Brahim, D. Narinesingh and A. G. Elie, Anal. Chim. Acta, 2001, 448, 27–36 CrossRef CAS.
- S. Singh, A. Chaubey and B. D. Malhotra, Anal. Chim. Acta, 2004, 502, 229–234 CrossRef CAS.
- Y. Li, H. Bai, Q. Liu, J. Bao, M. Han and Z. Dai, Biosens. Bioelectron., 2010, 25, 2356–2360 CrossRef CAS PubMed.
- M. Zhang, R. Yuan, Y. Chai, C. Wang and X. Wu, Anal. Biochem., 2013, 436, 69–71 CrossRef CAS PubMed.
- J. Yang, H. Lee, M. Cho, J. Nam and Y. Lee, Sens. Actuators, B, 2012, 171, 374–379 CrossRef.
- P. Gomathi, D. Ragupathy, J. H. Choi, J. H. Yeum, S. C. Lee, J. C. Kim, S. H. Lee and H. D. Ghim, Sens. Actuators, B, 2011, 153, 44–49 CrossRef CAS.
- A. Umar, M. M. Rahman, M. Vaseem and Y. B. Hang, Electrochem. Commun., 2009, 11, 118–121 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |