Hydrothermal synthesis of a Ba and Mg co-doped Bi12GeO20 photocatalyst with enhanced visible light catalytic activity
Abstract
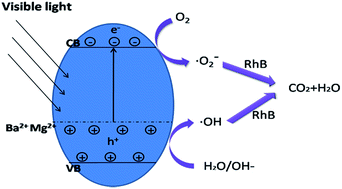
Visible-light-activated (Ba,Mg)-codoped Bi12GeO20 has been successfully synthesized through a one-step hydrothermal method. The as-prepared samples were characterized by X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM), high-resolution transmission electron microscopy (HRTEM), X-ray photoelectron spectroscopy (XPS), UV-vis diffuse reflectance spectra (DRS) and photoluminescence spectroscopy (PL). XPS study suggests that barium and magnesium were introduced into the Bi12GeO20 crystal successfully. The band gap of (Ba,Mg)-codoped Bi12GeO20 was greatly reduced in comparison with the pure and single-doped Bi12GeO20. (Ba,Mg)-codoped Bi12GeO20 showed higher photocatalytic activity than pure Bi12GeO20 and single-doped Bi12GeO20 in the photodegradation of Rhodamine B (RhB) aqueous solution under visible light irradiation. Photogenerated holes were the dominating active species, with the secondary and minor factors of hydroxyl radical and superoxide radical in the photodegradation process of (Ba,Mg)-codoped Bi12GeO20. For three cycles, the co-doped Bi12GeO20 exhibited high stability.

 Please wait while we load your content...
Please wait while we load your content...