Open Access Article
Open Access ArticleGas hydrates model for the mechanistic investigation of the Wittig reaction “on water”†
Khurshid
Ayub
*ad and
Ralf
Ludwig
*abc
aUniversität Rostock, Institut für Chemie, Abteilung für Physikalische Chemie, Dr.-Lorenz-Weg 1, 18059 Rostock, Germany. E-mail: ralf.ludwig@uni-rostock.de; Fax: +49 381 498 6524; Tel: +49 381 498 6517
bFaculty of Interdisciplinary Research, Department “Science and Technology of Life, Light and Matter”, University of Rostock, Rostock, Germany
cLeibniz-Institut für Katalyse an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
dDepartment of Chemistry, COMSATS Institute of Information Technology, University Road, Abbottabad, Pakistan 22060. E-mail: khurshid@ciit.net.pk
First published on 19th February 2016
Abstract
Theoretical mechanistic details for “on water” Wittig reaction of a stabilized ylide with benzaldehyde are presented and compared with a similar reaction under neat conditions. A gas hydrate structure consisting of 20 water molecules has been applied as a water surface for the reaction. The model is chosen to capture non-bonding interactions over a larger area in order to better account for the “on water” effect. The calculated acceleration for the cis-selective Wittig reaction is more than that for the trans-selective Wittig reaction. The “on water” acceleration for the Wittig reaction is due to greater number of non-bonding interactions in the transition state, compared to the starting material. The greater acceleration for the cis-selective Wittig over the trans-selective Wittig has been rationalized on the basis of non-bonding interactions in addition to hydrogen bonding. Besides accelerating the reaction, water also affects the pathway for the reaction. Decomposition of cisOP2 to alkene is estimated as a barrierless process. Moreover OP2 is more stable than OP1 for both cis and trans-selective Wittig reactions, opposite to what is observed for the neat reaction.
1. Introduction
Water is a green solvent, however its use in organic transformations had been quite rare until the discovery of Rideout and Breslow in 1980 (ref. 1) that the Diels–Alder reaction between non-polar substrates (eqn (1), Scheme 1) is accelerated significantly in homogeneous aqueous solution, when compared with solvent-free (neat) conditions or organic solvent-based reactions. The limited use of water was not only due to the classical solubility and reactivity consideration,2 but also because of the fear of hydrolysis of organic substrates, impeding catalytic activity, possible obstruction of functional groups, and side reactions caused by water.3 Later Sharpless4 coined the term “on water” for organic reactions which are accelerated by water but involve water insoluble reactants (eqn (2), Scheme 1). Sharpless has demonstrated that the reaction time for a heterogeneous mixture of reactants4,5 and water is much shorter (by a factor of 300) than the reaction time for a homogeneous mixture of reactant in water.Water may influence the chemical reaction in different ways depending whether the reaction is “in water” or “on water”.5 For a homogeneous reaction (in water), the generally operating effects are (1) hydrophobic effect6 (2) hydrogen bonding7 and (3) water polarity effects. The hydrophilic effect generally enhances the rate of the reaction whereas hydrogen bonding and polarity (water) may have additive or inverse effects to the hydrophobic effect. Some other effects reported in the literature include micellar catalysis,8 solvophobicity,9 cohesive energy density10 and ground state destabilization.11 A heterogeneous reaction (on water), involves trans phase interaction of water with transition states and reactants. Sharpless illustrated that unique properties at the macroscopic boundary between water and organic molecules are responsible for “on water” effects. The cases mentioned above are two extremes where organic compounds are either completely miscible with water or totally insoluble in water. However in majority of cases, there exists a broad spectrum of solubilities therefore the real operating mechanism under these conditions is a combination of “in water” and “on water” reaction mechanisms.
Although the early reports of acceleration of reaction “in water” and “on water” were on Diels–Alder reaction however the concept has been elaborated to a number of different examples. The concept of reaction “in” or “on-water” has been described in detail in recent books,12 reviews13 and articles.14 Sharpless4 also reported that Claisen rearrangement of 1-(4-chloronaphthyl)-1,1-dimethylallyl ether 7 (Scheme 2) in aqueous suspension was completed in 5 days at 23 °C. However the neat reaction took one additional day, and the reaction was even slower in organic solvents. The other reactions which are accelerated by water are “ene” reaction4,15 Claisen/Diels–Alder and more recently Wittig reaction16 in water where reactants have very low solubility in water. On water effect is observed not only in the acceleration of organic reactions but also in altering the regio- and stereoselectivities of the reactions. For example high level of endo selectivity is achieved through reaction “on water”. Similarly, Wittig reaction which is generally favorable for E-olefins from a stabilized ylide and aldehyde, E/Z selectivity is altered for reaction on water.
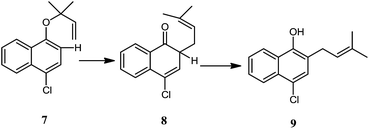 | ||
| Scheme 2 Claisen rearrangement of 1-(4-chloronaphthyl)-1,1-dimethylallyl ether on water, reported by Sharpless.4 | ||
Wittig reaction is a powerful tool for synthetic organic chemists to synthesize carbon carbon double bond with high stereoselectivity. Z-Alkenes are preferentially formed from non-stabilized ylides whereas E-alkenes are the major product from the reaction of stabilized ylides with aldehydes.17 The reaction of stabilized ylides with aldehydes is generally slow in non-polar solvents.18 Quite diverse strategies have been applied to accelerate/improve the reaction; increasing temperature,19 high pressures,20 irradiation with light21 or microwave, ionic solvents,22 silica gel,18 additives lithium salts23 Phase transfer catalyst,24 cyclodextrins25 and more recently water. The literature reveals both “in water” and “on water” acceleration for Wittig reaction. Bergdahl and coworkers3 have studied the “on water” Wittig reaction between the stabilized ylides with aldehydes and they demonstrated that the reaction is accelerated considerably but at the cost of E/Z selectivity. For example, the reaction of anisaldehyde 10 (Scheme 3) with a stabilized ylide (methoxycarbonyl methylene triphenylphosphorane) 11 delivered 36% yield with 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 E/Z selectivity whereas the same reaction under on water conditions furnished 81% yields of 12 in 1 hour with 76
18 E/Z selectivity whereas the same reaction under on water conditions furnished 81% yields of 12 in 1 hour with 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 E/Z ratio. Generally excellent results were obtained when hydrophobic groups were present (heterocycles). Moreover the authors have compared the reactivity of two different aldehydes (anisadehyde and 2-methoxycinnamaldehyde) with methoxycarbonyl methylene triphenylphosphorane. A general trend is reaction is accelerated in water however E/Z selectivity is decreased. Mostly regioselectivities were comparatively higher in organic solvents.
24 E/Z ratio. Generally excellent results were obtained when hydrophobic groups were present (heterocycles). Moreover the authors have compared the reactivity of two different aldehydes (anisadehyde and 2-methoxycinnamaldehyde) with methoxycarbonyl methylene triphenylphosphorane. A general trend is reaction is accelerated in water however E/Z selectivity is decreased. Mostly regioselectivities were comparatively higher in organic solvents.
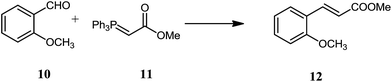 | ||
| Scheme 3 On water Wittig reaction between methoxycarbonyl methylene triphenylphosphorane and anisaldehyde. | ||
Reactions in water are not only important for experimentalists but they have gained interest from theoretical chemists as well especially in understanding the mechanisms for these accelerated transformations. For example, Jorgensen modelled the Diels–Alder reaction7b,c through ab initio methods using one explicit molecule of water and showed that multiple hydrogen bonding in the transition states are responsible for lowering the activation barrier about 3–5 kcal mol−1 which is in nice agreement with the experimental 4 kcal mol−1 for reaction in water. For on water conditions, recently Jung and Markus2 have applied the oil droplet model in order to explain the enhanced acceleration under “on water” conditions relative to “in water” conditions. They suggested that the enhanced acceleration can be rationalized when the transition state has more hydrogen bonding interactions with the surface water molecules than the reactants. The same oil droplet model has been used by Zhang and coworkers to explain the acceleration of aromatic Claisen rearrangement in water.26 Wittig reaction is accelerated on water however the stereoselectivity decreases (vide supra). This behavior has not been addressed in any theoretical study to date. Although the Wittig reaction has long history and even the mechanistic details of a non-catalyzed reaction were still under intense investigation through NMR27 and computational studies, although concluded to a single mechanism for salt free Wittig reaction very recently. With these facts, it becomes even more important to explore the mechanism of Wittig reaction on water, not only to address the acceleration on water but also the drop in stereoselectivity.
For mechanistic understanding, we propose here a different model in which instead of one or three water molecules, a gas hydrate of 20 water molecules is taken into account. We believe that this model is much superior to the previously taken models where a limited number of water molecules were taken into account.
The advantage of our model is not simply based on the larger number of water molecules (20 versus 3). In the (H2O)20 pentagonal dodecahedral structure all water molecules are bound as at the neat water surface. It is just perfect to mimic reactions “on water”. In contrast, small numbers of water molecules cannot represent the full cooperativity as present in the extended hydrogen bonding network of bulk water or at the water surface.28 Experimental and theoretical studies on gas phase clusters showed that full cooperativity is reached for the cyclic pentamer and hexamer, whereas cyclic trimers are strongly disfavored due to enthalpic and entropic reasons.28 Exactly twelve favorable cyclic structural motives are present in our dodecahedral water cluster. In isolated cyclic clusters only half of the OH groups are involved in hydrogen bonding, the other half is free. Such a situation is not known for water surfaces, where instead three of the two donor and two acceptors sites of each water molecule are part of the H-bond network. One free OH donor or one electron lone pair acceptor per water molecule points away from the surface and represent preferable reaction sites. Without taking boundary effects for flat water surfaces into account, the (H2O)20 cluster is the best model for investigating reaction “on water” because realistic H-bond strength, cooperativity and coordination numbers is taken into account.
We used the (H2O)20 pentagonal dodecahedral structure in earlier studies for showing the freezing of the “Bucky-ice” in the quantum cluster equilibrium model.29 Combined with the tetrakaidecahedral (H2O)24 or the heccacaidecahedral (H2O)28 this structure forms the principle building blocks of gas hydrates type-I and type II, respectively.30 Of course, gas hydrates are only stable at higher pressure and lower temperature and well supported by guest molecules. The most prominent species are methane hydrates for which giant natural methane deposits are expected in the deep ocean floor and in permafrost regions.30b Here, we want to show that these configurations can be used for studying reactions “on water” and that they better represent the water surface compared to a small number of not fully H-bonded water molecules applications.
2. Results and discussion
Water free mechanism
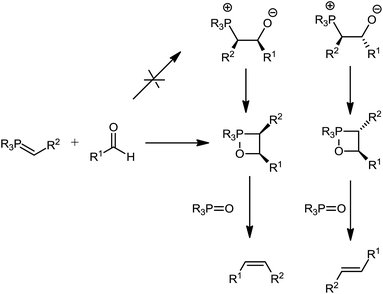
The mechanism of the Wittig reaction has faced several controversies over the past 60 years, since the discovery of the reaction.31 The mechanism remained the subject of intense debates and it can be illustrated by the fact that Johnson32 has shown eight different mechanisms that had been proposed at different times over the past six decades. This is one the great long standing investigation which has been addressed by both theoreticians and experimentalists. The controversies appeared due to improper addressing of all the contributing factors associated with the Wittig reaction.Wittig reaction can broadly be classified into two main categories; salt free Wittig reaction and salt mediated (mainly Li+). The mechanism of the latter is not well documented in the literature. Thanks to Gilheany and co-workers (experimental)31 and Harvey, Aggarwal and co-workers (theoretical),33 the mechanism of salt free Wittig reaction is now almost settled to a single mechanism for unstable, semi-stabilized and stabilized ylides where oxaphosphetanes are proposed as intermediates without the involvement of betaines. According to the mechanism (Scheme 4) the oxaphosphetane formation is irreversible, quite contrary to the early literature where the high E selectivity of Wittig reaction has been explained on the reversible nature of oxaphosphetane formation and thermodynamic control of the reaction. However it is interesting that a few examples where the oxaphosphetane formation is genuinely reversible belong to the class of non-stabilized ylides.31,33
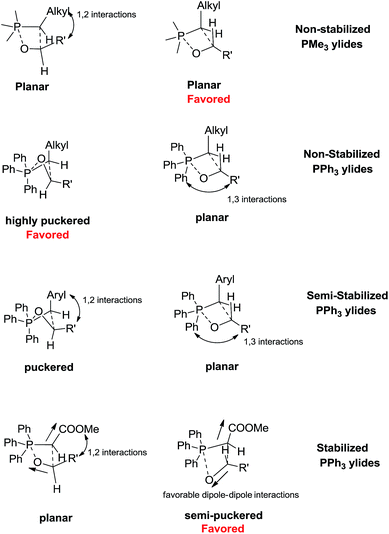
Despite the fact that the mechanism is same for non-stabilized, semi-stabilized and stabilized ylides, the reasons for the selectivities are different. The E/Z selectivity of the Wittig reaction is mainly explained by planar or puckered nature of the addition transition states. The degree of puckering depends on C–H⋯O hydrogen bonding (hydrogen bonding between a C–H of P-substituent and oxygen of the aldehyde), dipole–dipole interactions, and steric interactions between ylide and aldehyde substituents (1,2) and between phosphorus and aldehyde substituents (1,3). Except for 1,2 steric interaction, all other interactions are significantly affected by substituents on phosphorus.
The E/Z selectivity of the Wittig reaction from non-stabilized and semi-stabilized ylides is mainly explained on the basis of steric interactions (1,2 and 1,3); however, dipole–dipole interactions play key role in reactions from the stabilized ylides. For example, in the case of non- and semi stabilized ylides, methyl and methoxy substituents on phosphorus lead to planar and semi-puckered addition transition states, respectively. Therefore, 1,2 steric interactions operate in the favour of E-selectivity. For the planar geometries, 1,2 steric interactions are more pronounced in the cis TS. Therefore, the cis TS is less stable compared to the trans TS. The relatively higher stability of the trans TS delivers the E olefin. The situation is more complex with phenyl substituents where both puckered and planar transition states are observed. The selectivity is generally governed by a complex interplay of 1,2 and 1,3 steric interactions. The Wittig reaction from non-stabilized triphenyl phosphonium ylides proceeds through cis-puckered and trans planar transition states. Low 1,3 steric interaction combined with better solvation render the cis puckered TS more favourable (Z-selective). For semi-stabilized triphenyl phosphonium ylides, the cis transition state is less puckered therefore 1,2 steric interaction play significant role in addition to 1,3 steric interaction. Therefore, the Z-selectivity is reduced (E/Z mixture). The E-selectivity of the Wittig reaction from the stabilized ylides is mainly explained by dipole–dipole interactions between the reactants. The recent theoretical work illustrates that transition states for trans addition from stabilized ylides is puckered whereas the cis addition TS is planar (to avoid unfavourable electrostatic interactions). The planar structure of the cis TS is unfavourable due to increased 1,2 interactions. The dipole–dipole interaction is favourable (dipoles orient opposite to one another) for the trans addition therefore, E-selectivity is observed. The electrostatic origin of E-selectivity from stabilized ylides is contrary to what was originally proposed by Vedejs.34,35 According the earlier model proposed by Vedejs, the addition transition states from stabilized ylides are late in nature, and therefore non flexible. The planar trans TS is therefore more favourable due to reduced 1,2 steric interactions, compared to the cis TS.34,35
It is worth mentioning here that in this study, we have truncated the system from the original report of Bergdahl and coworkers.3 The ortho methoxy group on the aldehyde is removed, and the phenyl groups on the phosphorus of the ylide are replaced with methyl groups, in order to reduce the computational cost. This truncation is believed not to affect our study aimed at investigating on water mechanism of the Wittig reaction. The steric and electronic effects (if any) from phenyl and methoxy groups are believed to be comparable for both cis and trans product formation and therefore not expected to affect the energy profile significantly. To prove that the replacement of phenyl by methyl groups on the phosphorus of the ylide has only minor effects on the energy profiles, we have also investigated the energy profile for water free trans-selective Wittig reaction of triphenylphosphonium ylide, and compared with the energy profile of the same reaction “on water”. Moreover, we have also calculated the activation barrier for cis and trans addition of triphenylphosphonium ylide on benzaldehyde. As shown in the ESI,† the energy profiles are comparable. The reactions for both ylides show similar acceleration behaviour on water.
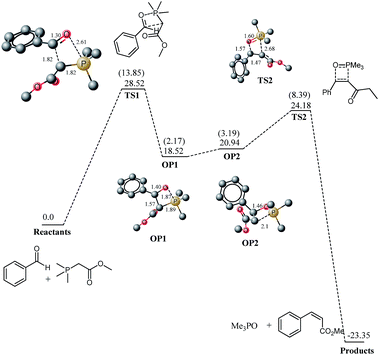
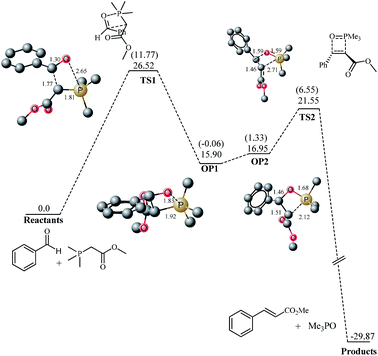
For a true comparison for reaction on water, an exactly identical system must have been theoretically studied in neat conditions as well. Fortunately Aggarwal, Harvey and coworkers33,36 have theoretically studied the reaction under neat and THF solvent conditions. However they reported only the results based on total energies without any zero point correction. We believe that Gibbs free energies are more meaningful for reactions involving reaction between more than one species because entropic factors are included in Gibbs free energy. We have calculated frequencies on the structures optimized by Aggarwal and coworkers and the results here are described and compared with “on water” study in terms of Gibbs free energy. The transition states were reported at barrier of 14.52 and 11.58 kcal mol−1 (total energies) for the cis and trans oxaphosphetane formation, respectively from the ylide and benzaldehyde. However the Gibbs free energies are 28.52 and 26.51 kcal mol−1 for the cis and trans oxaphosphetane formation respectively. In the transition state for cis oxaphosphetane formation TS1 (Fig. 2), C–C and P–C bond lengths are 1.82 A (Fig. 2) whereas the O–P bond length is 2.61 angstroms. One can observe that in the transition state C–C is formed considerably which indicates the late nature of the transition state, and it is consistent with the reports of Harvey and coworkers that the transition states from stabilized ylides and aldehdydes are late in nature. For the transition state leading to trans oxaphosphetane (Fig. 3), bond lengths are not much different. Since the ylides are stabilized therefore the formation of oxaphosphetane is endergonic by 18.52 (2.17) and 15.90 (−0.06) kcal mol−1 for the cis and trans oxaphosphetane formation, respectively. Oxaphosphetanes from non- and semi-stabilized ylides are lower in energy than the reactants (exothermic reaction) whereas oxaphosphetanes from stabilized ylides are higher in energy (endothermic).
The oxaphosphetane generated from the Wittig addition OP1 undergoes conformational changes to generate oxaphosphetanes-2 OP2 and the thermodynamic cost for the step is 2.42 (cis) and 1.05 (trans) kcal mol−1. Characteristic bond lengths for the intermediates and transition states are shown in Fig. 1 and 2. Transition states for retero (2 + 2) are located at barrier of 3.24 and 4.60 kcal mol−1 from the cis and trans oxaphosphetane-2, respectively.
Reaction “on water”
For the study of Wittig reaction on water we have chosen gas hydrates where twenty water molecules are used a model. The gas hydrates describe hydrogen bonding interactions in reactants and transition states more accurately because a number of water molecules are present all round to encompass the maximum number of interactions. This fact has been described to some extent by Zhang and coworkers in their study of benzene Claisen rearrangement where three water molecules provide much better illustration of hydrogen bonding than a single water molecule, and the result in the former case were very close to the experiment. We have extended the concept to twenty water molecules with the belief that the results will be further improved.Binding energy of ylide with water cluster
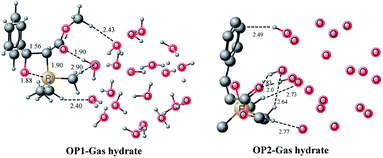
Our study for Wittig reaction “on water” begins with the formation of a complex between the ylide and gas hydrate. Gas hydrate complexes containing both ylide and benzaldehyde are insignificant in this study, and a brief discussion can be found in the ESI.† Since we proposed that 20 water molecule gas hydrate should better represent water surface for the reaction then one necessary condition for the validity of our hypothesis is that the gas hydrate structure is not affected significantly during the reaction. Therefore, immediately after complexation of the ylide with the gas hydrate, the structure of the complex (Fig. 4, right) was analyzed and compared with the gas hydrate before complexation (Fig. 4, left). The complexation of the ylide with the gas hydrate does not significantly affect the structure of the latter. One can observe that the pentagonal motifs, characteristic of the gas hydrate, are mostly retained except one pentagon which is transformed into an envelope like structure (shown by a directed arrow). But this pentagon is far away from the reaction center therefore this slight perturbation in structure of the gas hydrate does not influence the validity of our hypothesis. The ylide–gas hydrate complex is 17.59 kcal mol−1 more stable than the reactants. However the free energy of complexation is only −0.22 kcal mol−1. The high stability of the ylide–water-20 complex is due to multiple hydrogen bonding interactions (Fig. 5). In Fig. 5a, unnecessary hydrogen atoms are removed for clarity however Fig. 5b displays all hydrogen bonding interaction including inside the gas hydrates, and between the gas hydrate and the ylide. The structures of the gas hydrates have already been discussed in the literature in fair detail therefore only interactions with ylide are discussed here.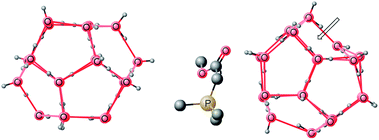 | ||
| Fig. 4 Illustration of pentagonal motifs of (H2O)20 gas hydrate before and after binding with ylide. | ||
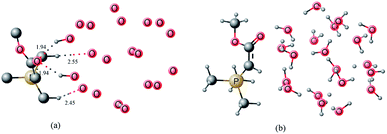 | ||
| Fig. 5 Gas hydrate–ylide complex (a) unimportant hydrogen atoms removed (b) all hydrogen atoms included. | ||
The carbonyl oxygen of the ester moiety in the ylide acts as hydrogen bond acceptor from two water molecules in the gas hydrates. The bond distances are 1.94 angstrom for both. Protons from two methyl groups of PMe3 group behave as hydrogen bond donor for gas hydrates. The interaction distances are 2.45 and 2.55 Å (Fig. 5a). These interaction are relatively weak compared to the interactions from the carbonyl oxygen, and this may be primarily attributed to the in space orientation of the ylide on the gas hydrate surface. Hydrogen bonding interactions between the ylide and gas hydrate also affects the hydrogen bonding interaction inside the gas hydrate (vide supra) therefore the binding energies can be used to measure the strength of hydrogen bonding established between the ylide and gas hydrates.
cis addition (2 + 2)
The transition states for “on water” Wittig reaction has been located for both cis and trans addition. A transition state for the cis addition of ylide–water complex TS1 (Fig. 6 and 7) to benzaldehyde is located at a barrier of 26.24 kcal mol−1 (Gibbs free energy of activation) and this is about 2.3 kcal mol−1 lower than the free energy of activation for the cis addition without water (neat). The lower activation barrier under “on water” conditions is very much consistent with the experimental observations. The non-bonding interactions of Wittig complex with gas hydrate are changed considerably from the interaction in the starting complex. The transition state shows more hydrogen bonding interaction when compared with the starting material and it supports the earlier concept by Sharpless4 that more hydrogen bonding interactions in the transition state compared to the starting materials lead to low activation barrier for the reaction carried out in or on water. The stability of the gas hydrate during the reaction is also investigated at the stage of TS1, by looking into pentagonal motifs of the gas hydrates. It can be easily seen in Fig. 7b, that the gas hydrate component retains most of its pentagons except one which is influenced by the approach of the aldehyde (may be a steric effect).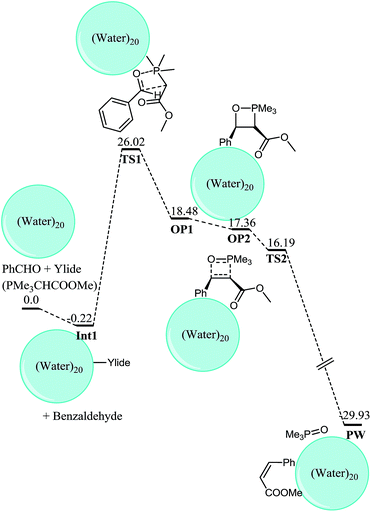 | ||
| Fig. 6 Gibbs free energy profile for cis-selective Wittig reaction on water, all energies are Gibbs free energies relative to benzaldehyde, ylide and water 20 molecule. | ||
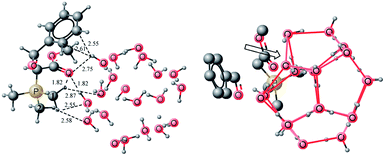 | ||
| Fig. 7 TS1 for cis Wittig addition (a) illustrating non-bonding interactions (b) illustration of pentagonal motifs of the gas hydrates. | ||
Two hydrogen atoms on the methyl carbon 1 (see Fig. 7) act as hydrogen donor to gas hydrate in the transition state, compared to one in the starting material. The H⋯O bond lengths are 2.55 and 2.87 Å. However the methyl 2 of PMe3 (see Fig. 7) has only one hydrogen bond interaction with the gas hydrate (2.58 Å), both in the transition state and the starting ylide water complex. The increased number of interactions from carbon 1 (see Fig. 7) may be considered as a consequence of the approach of the aldehyde. With the approach of the aldehyde, the change in the geometry of phosphorus causes the methyl groups to come close in space among them as well close to the gas hydrate. However the hydrogen bond acceptor interactions of the carbonyl oxygen with water molecules are slightly diminished compared to the starting material. The O⋯H bond lengths are 1.82 and 2.75 Å. A hydrogen from C1 of PMe3 acts as a hydrogen bond donor for the carbonyl oxygen (1.82 Å) which compensates for the diminished interaction of the carbonyl oxygen with the hydrogens of the gas hydrates. In the transition state, some interesting interactions are also observed besides the above mentioned hydrogen bonding interactions. The benzene moiety of benzaldehyde interacts with the hydrogen atoms of the water. A hydrogen atom from the gas hydrate water molecule interacts with two carbons of benzaldehyde (2.55 and 2.61 Å). Although significant interactions between gas hydrate and transition states are observed however the nature of the transition state and associated bond lengths are not affected much. For example, C–C bond being generated has bond length of 1.81 Å in both transition states (neat and on water). A slight difference in the bond length of P–O is observed between neat (2.61 Å) and on water (2.56 Å). The (2 + 2) addition of ylide–water complex with aldehyde is endergonic (ER = 18.7 kcal mol−1 (free energy of reaction), whereas the energy of reaction 3.82 kcal mol−1). The reaction is endergonic and it is consistent with the reports of Harvey, Aggarwal and coworkers33,36 that (2 + 2) addition of benzaldehyde and ylide in Wittig reaction is endothermic for stabilized ylides. The intermediates generated from the addition, OP1–gas hydrate (written as OP1 and so for other oxaphosphetanes in this discussion) is an oxaphosphetane. Bond distance for the C–C bond generated in the Wittig reaction is 1.56 Å and O–P bond is 1.88 Å. The C–P bond distance is 1.90 Å. All these bond lengths are characteristic of an oxyphosphatine. Non-bonding interactions are considerably reduced in the product when compared with the transition state. For example, non-bonding interactions between the phenyl ring and water molecules are absent altogether. Moreover the carbonyl oxygen of the ester moiety is an acceptor of only one hydrogen bond (1.90 Å) between the complex and gas hydrate compared to two for TS1. However the interactions between methyls of PMe3 and gas hydrate structure are almost similar.
Single point energy calculations are performed at higher level of theory to refine the free energies of activation (see ESI†). The results from higher level are consistent with the results from B3LYP/6-31G(d) that the cis Wittig addition is accelerated on the surface of water. The calculated acceleration effect for cis Wittig reaction at B3LYP/6-311++G(d,p) is much higher (about 5 kcal mol−1) than then one predicted at B3LYP/6-31G(d). The calculations have also been performed at Minnesota functional M05-2X (with 6-31G(d)) which is well known to account for non-bonding interactions. The results here also suggest much pronounced acceleration of cis Wittig reaction on water (see ESI†). These results suggest that B3LYP/6-31G(d) level of theory is probably illustrating the minimum limit of acceleration of Wittig reaction.
The oxaphosphetane OP1–water 20 undergoes slight changes in the geometry prior to it decomposition into alkene however we have not modeled the transition state for this step because it is almost insignificant in our studies of “on water” Wittig reaction mainly because of the fact that the activation barrier for this process is insignificant. The OP2 generated is more stable than OP1 and this behavior is opposite to the observed for neat reaction. In reactions without water, OP2 is 2.42 kcal mol−1 higher in energy than OP1. The higher stability of OP2–water complex over OP1 complex may be rationalized on geometric analysis. OP2 has more non-bonding interactions with water-20 complex compared to OP1–water complex.
For example, carbonyl oxygen is one hydrogen bond acceptor in OP1 whereas in OP2, it accepts two hydrogen bonds from the gas hydrate (Fig. 8 for details). Moreover the hydrogen bond are 2.0 and 1.83 Å in OP2 compared to 1.90 Å in OP1. In addition, methyl groups on PMe3 are three hydrogen bonds donor in OP2 compared to two hydrogen bonds donor in OP1. More interestingly, the phenyl ring of the aldehyde fragment is far away from the gas hydrate in OP1 whereas this phenyl ring is in close proximity to a hydrogen from the gas hydrate and has a favorable interaction between a meta carbon and a hydrogen from the gas hydrates. The enhanced interaction between the Wittig complex with the gas hydrate in OP2 compared to OP1, make the former thermodynamically more stable. All attempts to locate a transition state for retro (2 + 2) from OP2 met with failure. However we isolated a structure during the optimization of TS2 with RMS gradient 4.2 × 10−4 which has the right frequency for the retro (2 + 2) but this structure now called as TS2 is lower in energy compared to OP2 which indicates that this process is a barrierless process. A low activation barrier is generally expected for retro (2 + 2) in cis oxaphosphetane however its barrierless nature has not been reported to date, to the best of our knowledge. The transition state may be taken as a stationary point on PES if the convergence criteria reported by Aggarwal, Harvey and co-worker is taken into account. In their report of mechanistic study of Wittig reaction, structures with RMS gradient of 0.0015 were taken as stationary point on PES (they used loose optimization under Jaguar formalism).
The product of the reaction is a complex in which phosphoxide and Wittig product (olefin) are bound to gas hydrate, and this structure is called product water complex (PW). The PW complex is highly stable and lies about 47.29 kcal mol−1 lower in energy than the OP2–hydrate complex. The high thermodynamic stability of the PW is also a factor for difficulties in locating TS2, and even OP2 because any attempt leads to the optimization of PW. Since the reaction is thermodynamically highly exergonic therefore the transition state is an early transition state according to the Hammond Leffler postulate.
trans addition
For the trans (2 + 2) addition of benzaldehyde and ylide under on water conditions, a transition state has been located at a barrier of 25.12 kcal mol−1 from the ylide water complex. This activation barrier is about 1.4 kcal mol−1 lower compared to the activation barrier for the neat reaction (without any water molecules, 26.52 kcal mol−1). A few important hydrogen bonding interactions in the transition state are shown in Fig. 9. A proton from the methyl of the ester moiety acts as hydrogen donor for a water molecule in the gas hydrate. The carbonyl oxygen accepts only one hydrogen bond from the hydrate whereas the transition state for the cis addition displayed two interactions from the carbonyl oxygen. The other interactions were almost comparable between the cis and trans transition states. An oxaphosphetane is generated from the trans addition of ylide and benzaldehyde and the reaction is endergonic by 14.23 kcal mol−1 compared to 18.48 kcal mol−1 for the formation of cis oxaphosphetane. In the case of trans addition, no significant difference in non-bonding interaction is observed between TS1 and oxaphosphetane. The number of interactions and interacting atoms are same in TS1 and oxyphosphatine. The bond lengths at the reaction center change, for example, C–C bond generated has bond length of 1.74 Å in the transition state which reduced to 1.54 Å in OP1. Similarly P–O bond reduces in length from 2.64 Å in TS to 1.84 Å in OP1.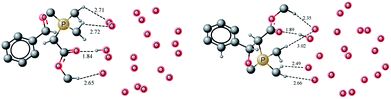 | ||
| Fig. 9 Optimized geometries of transTS1 and transOP1. All bond lengths are in Angstrom. Hydrogen atoms are removed for clarity. | ||
Although the calculations at B3LYP/6-31G(d) suggest acceleration of trans Wittig reaction as well, but the results from B3LYP/6-311++G(d,p) and M05-2X/6-31G(d) are quite opposite. The level of theories suggest deceleration of trans Wittig reaction (see ESI†). These calculations would suggest that E/Z ratio should be small because cis Wittig is accelerated but trans is decelerated (contrary to the experimental observations). These results forced to look deep into the experimental conditions for Wittig reaction. We could realize that they added HCl to the water reactions before work up! Their idea was to make sure no more Wittig reaction occurred during work up in the organic solvent extraction. This was a good idea in principle but acid could have isomerised a portion of the Z-isomer to the E-isomer. So their reported E/Z ratios could be artificially high in E-isomer.
OP1 gets transformed into OP2 and the process is thermodynamically favorable, quite similar to the case of cisOP2 from OP1. The higher stability of OP2 relative to OP1 is common for both cis and trans-selective Wittig reaction on water. The transOP2 is of higher energy compared to OP1 (by 1.39 kcal mol−1) in neat conditions. The transOP2 is a stable species on the potential energy surface. A transition state for the retro (2 + 2) from OP2 has been located at a barrier of 1.15 kcal mol−1. Although this activation barrier is negligible for trans retro (2 + 2) but still it represents a marked difference from the behavior of cisOP2 where the cleavage has been shown to be a barrierless process. In the transition state the P–C bond which is being broken has bond length of 2.21 Å and the C–O bond is 1.46 Å (Fig. 10). The retro (2 + 2) reaction from OP2 to form E product complex PW is thermodynamically highly favorable (49.75 kcal mol−1). The overall process is also thermodynamically favorable one by 35.2 kcal mol−1 (Fig. 11).
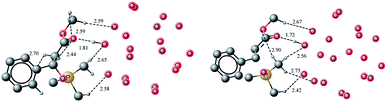 | ||
| Fig. 10 Optimized geometries of transOP2 and TS2, hydrogen atoms are removed for clarity and all bond lengths are in Angstroms. | ||
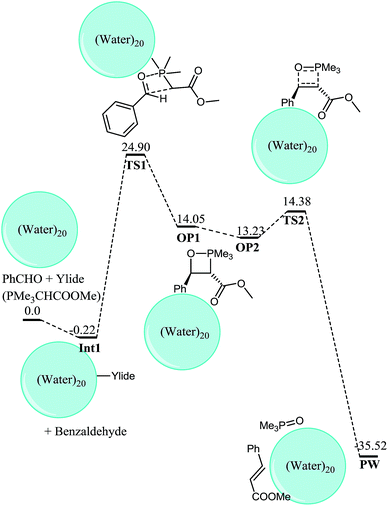 | ||
| Fig. 11 Gibbs free energy profile for trans-selective Wittig reaction on water, all energies are Gibbs free energies relative to benzaldehyde, ylide and water 20 molecule. | ||
3. Conclusions
In summary, we have shown that gas hydrate model consisting of 20 water molecules nicely illustrates the acceleration of the Wittig reaction on water. Water not only affects the reaction rates for both cis and trans Wittig reaction but also affects the relative stabilities of different intermediates during the course of the reaction. Low activation barrier for the rate determining formation of oxaphosphetane illustrates that Wittig reaction for the formation of both cis and trans olefins is accelerated, however the former is accelerated more than the latter. Another remarkable difference between the neat and “on water” reaction is the higher stability of OP2 than OP1 in the latter case whereas the reverse is true for neat reaction. For the cis-selective Wittig reaction, the retro (2 + 2) from oxaphosphetane OP1 is a barrier less process however a barrier of 1.38 kcal mol−1 is the calculated from OP2 (trans).4. Experimental section
All calculations were performed with Gaussian 09.37 Geometries of the structures were optimized without any symmetry constraints at hybrid B3LYP method of DFT at 6-31G* basis set.38 The B3LYP method is quite effective at predicting the behavior and geometries of neutral39 and charged species40 including study of reaction in and on water. The method has also been used for the study of Diels–Alder and aromatic Claisen26 rearrangement under on water conditions. Each optimized structure was confirmed by a frequency analysis at the same level of theory as a true minimum (no imaginary frequency) or a transition state (one imaginary frequency). The imaginary frequencies were also evaluated to confirm that their associated eigenvector correspond to the motion along the reaction coordinates. The reported energies are Gibbs free energies and are in kcal mol−1. Total energies and zero point energies can be found in the ESI.†Acknowledgements
This work has been supported by the project “Light2Hydrogen” of the BMBF and the project “Nano4Hydrogen” of the ESF and the state of Mecklenburg-Vorpommern. K. A. thanks COMSATS Institute of Information Technology and Higher Education Commission of Pakistan for support.References
- D. C. Rideout and R. Breslow, Hydrophobic acceleration of Diels–Alder reactions, J. Am. Chem. Soc., 1980, 102(26), 7816–7817 CrossRef CAS.
- Y. Jung and R. Marcus, On the theory of organic catalysis “on water”, J. Am. Chem. Soc., 2007, 129(17), 5492–5502 CrossRef CAS PubMed.
- A. El-Batta, C. Jiang, W. Zhao, R. Anness, A. L. Cooksy and M. Bergdahl, Wittig reactions in water media employing stabilized ylides with aldehydes. Synthesis of α,β-unsaturated esters from mixing aldehydes, α-bromoesters, and Ph3P in aqueous NaHCO3, J. Org. Chem., 2007, 72(14), 5244–5259 CrossRef CAS PubMed.
- S. Narayan, J. Muldoon, M. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, “On water”: Unique reactivity of organic compounds in aqueous suspension, Angew. Chem., Int. Ed., 2005, 44(21), 3275–3279 CrossRef CAS PubMed.
- R. N. Butler and A. G. Coyne, Water: Nature's Reaction Enforcer–Comparative Effects for Organic Synthesis “In-Water” and “On-Water”, Chem. Rev., 2010, 110(10), 6302–6337 CrossRef CAS PubMed.
- R. Breslow, Hydrophobic effects on simple organic reactions in water, Acc. Chem. Res., 1991, 24(6), 159–164 CrossRef CAS.
- (a) O. Acevedo and W. L. Jorgensen, Understanding rate accelerations for Diels–Alder reactions in solution using enhanced QM/MM methodology, J. Chem. Theory Comput., 2007, 3(4), 1412–1419 CrossRef CAS PubMed; (b) J. F. Blake and W. L. Jorgensen, Solvent effects on a Diels–Alder reaction from computer simulations, J. Am. Chem. Soc., 1991, 113(19), 7430–7432 CrossRef CAS; (c) J. F. Blake, D. Lim and W. L. Jorgensen, Enhanced hydrogen bonding of water to Diels–Alder transition structures. Ab initio evidence, J. Org. Chem., 1994, 59(4), 803–805 CrossRef CAS; (d) J. Chandrasekhar, S. Shariffskul and W. L. Jorgensen, QM/MM simulations for Diels–Alder reactions in water: contribution of enhanced hydrogen bonding at the transition structure to the solvent effect, J. Phys. Chem. B, 2002, 106(33), 8078–8085 CrossRef CAS; (e) T. R. Kelly, P. Meghani and V. S. Ekkundi, Diels–Alder reactions: Rate acceleration promoted by a biphenylenediol, Tetrahedron Lett., 1990, 31(24), 3381–3384 CrossRef CAS; (f) I. Willner, E. Katz, A. Riklin and R. Kasher, Mediated electron transfer in glutathione reductase organized in self-assembled monolayers on gold electrodes, J. Am. Chem. Soc., 1992, 114(27), 10965–10966 CrossRef CAS.
- R. Breslow, U. Maitra and D. Rideout, Selective Diels–Alder reactions in aqueous solutions and suspensions, Tetrahedron Lett., 1983, 24(18), 1901–1904 CrossRef CAS.
- H.-J. Schneider and N. K. Sangwan, Diels–Alder reactions in hydrophobic cavities: a quantitative correlation with solvophobicity and rate enhancements by macrocycles, J. Chem. Soc., Chem. Commun., 1986, 24, 1787–1789 RSC.
- J. J. Gajewski, A semitheoretical multiparameter approach to correlate solvent effects on reactions and equilibria, J. Org. Chem., 1992, 57(20), 5500–5506 CrossRef CAS.
- W. Blokzijl and J. B. Engberts, Hydrophobic effects. Opinions and facts, Angew. Chem., Int. Ed. Engl., 1993, 32(11), 1545–1579 CrossRef.
- (a) P. Grieco and T. Loh, Organic Synthesis in Water, Angew. Chem., Int. Ed., 1998, 37(16), 2279–2279 Search PubMed; (b) U. M. Lindstrom, Organic reactions in water: principles, strategies and applications, Wiley. com, 2008 Search PubMed.
- (a) A. Chanda and V. V. Fokin, Organic synthesis “on water”, Chem. Rev., 2009, 109(2), 725–748 CrossRef CAS PubMed; (b) C.-J. Li, Organic reactions in aqueous media with a focus on carbon-carbon bond formations: a decade update, Chem. Rev., 2005, 105(8), 3095–3166 CrossRef CAS PubMed; (c) C. J. Li, Organic reactions in aqueous media-with a focus on carbon-carbon bond formation, Chem. Rev., 1993, 93(6), 2023–2035 CrossRef CAS; (d) U. M. Lindström, Stereoselective organic reactions in water, Chem. Rev., 2002, 102(8), 2751–2772 CrossRef.
- (a) J. B. Engberts and M. J. Blandamer, Understanding organic reactions in water: from hydrophobic encounters to surfactant aggregates, Chem. Commun., 2001, 18, 1701–1708 RSC; (b) J. E. Klijn and J. B. Engberts, Organic chemistry: Fast reactions ‘on water’, Nature, 2005, 435(7043), 746–747 CrossRef CAS PubMed; (c) S. Otto and J. B. Engberts, Diels–Alder reactions in water, Pure Appl. Chem., 2000, 72(7), 1365–1372 CrossRef CAS.
- K. Nicolaou, H. Xu and M. Wartmann, Biomimetic total synthesis of gambogin and rate acceleration of pericyclic reactions in aqueous media, Angew. Chem., 2005, 117(5), 766–771 CrossRef.
- J. Dambacher, W. Zhao, A. El-Batta, R. Anness, C. Jiang and M. Bergdahl, Water is an efficient medium for Wittig reactions employing stabilized ylides and aldehydes, Tetrahedron Lett., 2005, 46(26), 4473–4477 CrossRef CAS.
- (a) O. I. Kolodiazhnyi, Phosphorus ylides, Wiley. com, 2008 Search PubMed; (b) A. Maercker, The Wittig reaction, Wiley Online Library, 1965 Search PubMed; (c) B. E. Maryanoff and A. B. Reitz, The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects, Chem. Rev., 1989, 89(4), 863–927 CrossRef CAS.
- V. J. Patil, Wittig reactions in the presence of silica gel, Tetrahedron Lett., 1996, 37(8), 1281–1284 CrossRef CAS.
- G. Fodor and I. Tőmőskőzi, The reaction of carbethoxymethylenetriphenylphosphorane with ketones, Tetrahedron Lett., 1961, 2(16), 579–582 CrossRef.
- (a) N. S. Isaacs and G. N. El-Din, The application of high pressure to some difficult Wittig reactions, Tetrahedron Lett., 1987, 28(19), 2191–2192 CrossRef CAS; (b) A. Nonnenmacher, R. Mayer, H. Plieninger and X. I. I. Hochdruckversuche, Über die Anwendung von hohem Druck bei Wittig-Reaktionen mit resonanzstabilisierten Yliden, Liebigs Ann. Chem., 1983, 1983(12), 2135–2140 CrossRef.
- (a) A. Spinella, T. Fortunati and A. Soriente, Microwave accelerated Wittig reactions of stabilized phosphorus ylides with ketones under solvent-free conditions, Synlett, 1997, 1(01), 93–94 CrossRef; (b) J. Wu, H. Wu, S. Wei and W.-M. Dai, Highly regioselective Wittig reactions of cyclic ketones with a stabilized phosphorus ylide under controlled microwave heating, Tetrahedron Lett., 2004, 45(22), 4401–4404 CrossRef CAS; (c) C. Xu, G. Chen, C. Fu and X. Huang, The Wittig reaction of stable ylide with aldehyde under microwave irradiation: synthesis of ethyl cinnamates, Synth. Commun., 1995, 25(15), 2229–2233 CrossRef CAS.
- V. Le Boulaire and R. Grée, Wittig reactions in the ionic solvent [bmim][BF4], Chem. Commun., 2000, 22, 2195–2196 RSC.
- (a) a. E. Corey, D. A. Clark, G. Goto, A. Marfat, C. Mioskowski, B. Samuelsson and S. Hammarstroem, Stereospecific total synthesis of a “ slow reacting substance” of anaphylaxis, leukotriene C-1, J. Am. Chem. Soc., 1980, 102(4), 1436–1439 CrossRef; (b) S. Fliszar, R. Hudson and G. Salvadori, Note sur la catalyse acide de la réaction des phosphobétaïnes avec le benzaldéhyde, Helv. Chim. Acta, 1964, 47(1), 159–162 CrossRef CAS; (c) D. L. Hooper, S. Garagan and M. M. Kayser, Lithium cation-catalyzed Wittig reactions, J. Org. Chem., 1994, 59(5), 1126–1128 CrossRef CAS; (d) H. O. House, V. K. Jones and G. A. Frank, The Chemistry of Carbanions. VI. Stereochemistry of the Wittig Reaction with Stabilized Ylids1a, J. Org. Chem., 1964, 29(11), 3327–3333 CrossRef CAS; (e) D. Marriott and J. Bantick, 5(S),6(R)-5,7-dibenzoyloxy-6-hydroxyheptanoate ester: improved synthesis of a leukotriene intermediate, Tetrahedron Lett., 1981, 22(37), 3657–3658 CrossRef CAS; (f) C. Rüchardt, P. Panse and S. Eichler, Zum Mechanismus der Wittig-Reaktion mit Triaryl-alkoxycarbonylmethylen-phosphoranen, Chem. Ber., 1967, 100(4), 1144–1164 CrossRef.
- J. A. Stafford and J. E. McMurry, An efficient method for the preparation of alkylidenecyclopropanes, Tetrahedron Lett., 1988, 29(21), 2531–2534 CrossRef CAS.
- G. Westman, O. Wennerström and I. Raston, On the effect of cyclodextrin on the Z/E-selectivity of Wittig reactions with semistabilized ylides, Tetrahedron, 1993, 49(2), 483–488 CrossRef CAS.
- Y. Zheng and J. Zhang, Catalysis in the oil droplet/water interface for aromatic Claisen rearrangement, J. Phys. Chem. A, 2010, 114(12), 4325–4333 CrossRef CAS PubMed.
- F. Bangerter, M. Karpf, L. A. Meier, P. Rys and P. Skrabal, Observation of Pseudorotamers of Two Unconstrained Wittig Intermediates, (3RS,4SR)-and (3RS,4RS)-4-Cyclohexyl-2-ethyl-3,4-dimethyl-2,2-diphenyl-1,2λ5-oxaphosphetane, by Dynamic 31P NMR Spectroscopy: Line-Shape Analyses, Conformations, and Decomposition Kinetics, J. Am. Chem. Soc., 1998, 120(41), 10653–10659 CrossRef CAS.
- (a) T. Köddermann, F. Schulte, M. Huelsekopf and R. Ludwig, Die Bildung von Wasserclustern in einem hydrophoben Lösungsmittel, Angew. Chem., 2003, 115(40), 5052–5056 CrossRef; (b) T. Köddermann, F. Schulte, M. Huelsekopf and R. Ludwig, Formation of water clusters in a hydrophobic solvent, Angew. Chem., Int. Ed., 2003, 42(40), 4904–4908 CrossRef PubMed.
- (a) R. Ludwig, Wasser: von Clustern in die Flüssigkeit, Angew. Chem., 2001, 113(10), 1856–1876 CrossRef; (b) R. Ludwig, Water: from clusters to the bulk, Angew. Chem., Int. Ed., 2001, 40(10), 1808–1827 CrossRef CAS; (c) R. Ludwig and A. Appelhagen, Berechnung clathratähnlicher Wassercluster einschließlich eines Wasser-Buckminsterfullerens, Angew. Chem., Int. Ed., 2005, 44(5), 811–815 CrossRef CAS PubMed; (d) R. Ludwig and A. Appelhagen, Calculation of Clathrate-Like Water Clusters Including H2O-Buckminsterfullerene, Angew. Chem., Int. Ed., 2005, 44(5), 811–815 CrossRef CAS PubMed; (e) R. Ludwig and F. Weinhold, Quantum cluster equilibrium theory of liquids: Freezing of QCE/3-21G water to tetrakaidecahedral “Bucky-ice”, J. Chem. Phys., 1999, 110, 508 CrossRef CAS; (f) S. D. Belair, H. Hernandez and J. S. Francisco, J. Am. Chem. Soc., 2004, 126, 3024 CrossRef CAS PubMed.
- (a) D. Davidson, Clathrate hydrates, Water: A comprehensive treatise, 2, ed. F. Franks, Plenum, New York, 1973, pp. 115–234 Search PubMed; (b) E. Sloan, Clathrate Hydrates of Natural Gases, Marcel Dekker, New York, 1990 Search PubMed.
- (a) P. A. Byrne and D. G. Gilheany, Unequivocal Experimental Evidence for a Unified Lithium Salt-Free Wittig Reaction Mechanism for All Phosphonium Ylide Types: Reactions with β-Heteroatom-Substituted Aldehydes Are Consistently Selective for cis-Oxaphosphetane-Derived Products, J. Am. Chem. Soc., 2012, 134(22), 9225–9239 CrossRef CAS PubMed; (b) P. A. Byrne and D. G. Gilheany, The modern interpretation of the Wittig reaction mechanism, Chem. Soc. Rev., 2013, 42, 6670–6696 RSC.
- A. W. Johnson, W. C. Kaska, K. O. Starzewski and D. Dixon, Ylides and imines of phosphorus, Wiley, New York, 1993 Search PubMed.
- R. Robiette, J. Richardson, V. K. Aggarwal and J. N. Harvey, Reactivity and selectivity in the Wittig reaction: A computational study, J. Am. Chem. Soc., 2006, 128(7), 2394–2409 CrossRef CAS PubMed.
- (a) E. Vedejs, C. F. Marth and R. Ruggeri, Substituent Effects and the Wittig Mechanism: The Case for Stereospecific Oxaphosphetane Decomposition, J. Am. Chem. Soc., 1988, 110, 3940–3948 CrossRef CAS; (b) E. Vedejs and C. F. Marth, Mechanisms of the Wittig Reaction: The Role of Substituents at Phosphorus, J. Am. Chem. Soc., 1988, 110, 3948–3958 CrossRef CAS; (c) E. Vedejs and T. J. Fleck, Kinetic (Not Equilibrium) Factors Are Dominant in Wittig Reactions of Conjugated Ylides, J. Am. Chem. Soc., 1989, 111, 5861–5871 CrossRef CAS; (d) E. Vedejs and C. F. Marth, Mechanisms of the Wittig Reaction: Evidence against Betaine Intermediates, J. Am. Chem. Soc., 1990, 112, 3905–3909 CrossRef CAS.
- (a) E. Vedejs and M. J. Peterson, Stereochemistry and Mechanism in the Wittig Reaction, Top. Stereochem., 2007, 21, 1–157 Search PubMed; (b) B. E. Marynoff and A. B. Reitz, Chem. Rev., 1989, 89, 863–927 CrossRef.
- J. N. Harvey, Ab initio transition state theory for polar reactions in solution, Faraday Discuss., 2010, 145, 487–505 RSC.
- M. J. Frisch, T. G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Jr Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09 Revision C. 01, Gaussian Inc., Wallingford CT, 2010 Search PubMed.
- P. C. Haharan and J. A. Pople, The influence of Polarisation functions on Molecular Orbital Hydrogenation Energies, Theor. Chim. Acta, 1973, 28, 213 CrossRef.
- V. Specowius, F. Bendrath, M. Winterberg, K. Ayub and P. Langer, Synthesis of Functionalized Indolizines by Lewis Acid-Mediated Cyclocondensation of 3-(Pyridin-2-yl)-propiolates with Enones, Adv. Synth. Catal., 2012, 354(6), 1163–1169 CrossRef CAS.
- V. O. Iaroshenko, D. Ostrovskyi, K. Ayub, A. Spannenberg and P. Langer, Synthesis of 4-Trifluoromethylpyridines by [5 + 1] Cyclization of 3-Hydroxy-pent-4-yn-1-ones with Urea, Adv. Synth. Catal., 2013, 355(2–3), 576–588 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25747f |
| This journal is © The Royal Society of Chemistry 2016 |