Assessment of polydopamine coated magnetic nanoparticles in doxorubicin delivery†
Abstract
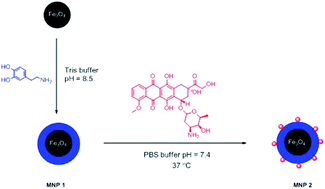
Magnetic nanoparticles (MNP) coated with bioinspired polydopamine (PDA) were obtained via a co-precipitation method and oxidative polymerization of dopamine. Nanoparticles were investigated by FTIR, TEM and SQUID. Loading capacity of anticancer drug doxorubicin was determined by UV-Vis spectroscopy. The nanocomposites exhibit a high drug loading capacity of 0.46 mg mg−1. Anticancer activity of the nanocomposites was proved in profound in vitro tests on HeLa cells. Cytotoxicity and internalization of nanoparticles were checked using various method, i.e. proliferation assay (WST-1), a two-colour fluorescence cell viability assay, and fluorescent and confocal microscopy.

 Please wait while we load your content...
Please wait while we load your content...