Cost-effective solar concentrators based on red fluorescent Zn(II)–salicylaldiminato complex†
Pierpaolo Mineia,
Elisabetta Fanizzabc,
Antonio M. Rodríguezd,
Ana B. Muñoz-Garcíad,
Paola Ciminoe,
Michele Pavone*d and
Andrea Pucci *fg
*fg
aScuola Normale Superiore, Piazza dei Cavalieri 7, I-56126 Pisa, Italy
bDipartimento di Chimica, Università degli Studi di Bari, Via Orabona 4, 70126 Bari, Italy
cCNR-Istituto per i Processi Chimico Fisici, UOS Bari, Via Orabona 4, 70126 Bari, Italy
dDipartimento di Scienze Chimiche, Università di Napoli Federico II, Complesso Universitario Monte Sant'Angelo Via Cintia 21, 80126 Napoli, Italy. E-mail: michele.pavone@unina.it
eDipartimento di Scienze Farmaceutiche Università degli Studi di Salerno, Via Ponte don Melillo, Fisciano, I-84084 Salerno, Italy
fDipartimento di Chimica e Chimica Industriale, Università di Pisa, Via Moruzzi 13, 56124 Pisa, Italy. E-mail: andrea.pucci@unipi.it
gINSTM, UdR Pisa, Via Moruzzi 13, 56124 Pisa, Italy
First published on 29th January 2016
Abstract
Sunlight concentration is a promising path to cost-effective photovoltaic (PV) technologies. Compared to standard concentrators based on geometrical optics, luminescent solar concentrators (LSCs) appear to be viable and convenient alternatives because sunlight concentration to PV occurs with diffuse light and there is no need for sun tracking or cooling apparatuses. In this study, we report on the optical efficiencies of luminescent solar concentrators (LSCs) based on poly(methyl methacrylate) (PMMA) thin films doped with a red-emitting zinc(II) complex of the D–A–D type ligand N,N′-bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile (ZnL). ZnL is attractive for use in LSC owing to its easy and cheap synthesis. ZnL in PMMA shows an emission band at 624 nm, a Stokes shift of 34 nm and an average QY of 23%, data comparable to those recorded in solution and efficiently predicted by DFT calculations. A study of ZnL/PMMA LSC yields optical efficiencies of 7%, which is comparable to those based on the near unity QY fluorophores such as Lumogen Red. These performances were attributed to the higher emission red-shift and larger Stokes shift of ZnL that prevent the loss of efficiencies due to self-absorption and possibly circumvent its lower QY.
Introduction
Since the beginning of solar power production, the concentration of solar radiation has been proposed as a solution to decrease the price of photovoltaic energy. Solar concentration is achieved by collecting the sun radiation incident on a large surface and redirecting it on a smaller area, thus allowing it to reduce the amount of photoactive materials, which has the largest impact on the final costs.1–3 There are mainly two types of solar concentrators, one based on geometrical optics (passive concentrators)4 and another group based on luminescent components (active solar concentrators).3,5 Solar power fields with passive concentrators made of parabolic mirrors and Fresnel lenses are already a reality because they take a large area of sunlight and direct it towards a specific spot by bending the rays of light and focusing them.6 This has the advantages of working for all wavelengths, because it depends on reflection rather than refraction, and not requiring any extreme materials properties.7 While capable of achieving extremely high concentrations (several hundred suns),8 the current technology suffers of some practical limitations such as size limit (it is very long compared to its diameter), dependence on the sunlight incidence angle, needs of large and heavy sun tracking systems and cooling apparatuses.9,10 These features have hindered the deployment of such technology in urban environments and, in the past decades, active luminescent solar concentrators (LSCs) have been proposed as a viable and convenient alternative to classic geometric concentrators.11 LSCs have several advantages as follows: the ability to work with diffuse light, light weight, reduced costs, and transparency are few examples.12,13 These last features make LSCs well suited to be implemented in modern building architectures, which make use of plenty of coloured windows and panels.14 Moreover, the use of commodity plastics and well consolidated and economic industrial processes for the preparation of LSCs offer an encouraging means to include solar energy to the built environment.The standard LSC device consists in a slab of transparent material (usually glass or polymer) doped with fluorescent dyes that absorb in the solar spectrum.14–16 The refractive index of the host is higher than the outer environment: total internal reflections are allowed to trap inside the LSC a large fraction of the dye emitted photons, which are thus collected and concentrated at the device edges wherein a PV module can be attached. In recent years, the study on PV devices based on LSC technology has been focusing on obtaining high power conversion efficiencies.13,17–26 However, prototype single-dye LSC coupled to commercial Si cells achieved to date have efficiencies no higher than 3%.14 Such low performance is mostly due to the many losses of such devices, due to both the physics of the phenomena and a not-yet-optimized fluorescent system.27
A simple approach for higher concentrations is to enhance the spectral window of absorption of the LSC, therefore increasing the number of available photons. To this end, multiple dye systems have long been proposed to cope for the narrow absorption characteristic of organic dyes as well as new design solutions.12,22,28 Noble metals nanoparticles29 and quantum dots have also been investigated for their broad absorption features although compatibility issues between the fluorophore and commercial matrices seldom arise.18,30–32 To the best of our knowledge, however, there are only a few examples of the use of metal complexes. Tris(8-hydroxyquinolinolate)aluminium(III) (Alq3) and platinum tetraphenyltetrabenzoporphyrin [Pt(TPBP)] have been employed effectively as robust photoemitters in stacked high-efficiency LSCs.22 Indeed, the organic metal–chelate complexes usually offer additional convenient features such as high thermal and optical stability,33,34 wide absorption range and luminescent properties.
For these reasons, a promising red-emitting zinc(II) complex of the donor–acceptor–donor (D–A–D) type ligand N,N′-bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile (H2L) was investigated (Fig. 1). One of the main appeals of this class of inorganic complexes is that molecular engineering permits systematically altering the spectroscopic and chemical properties. This chemical flexibility allows for the design of systems that respond to specific environmental variables. Zn(II) complexes bearing salicylaldiminato ligands have been particularly employed as emitters in organic optoelectronics,33,35,36 and also exhibit a broad range of eco-friendly catalytic37 and biological activities.38,39 It can be noted that considering the easy and cheap synthetic route, ZnL might represent the first example of a cost-effective red-emitting Schiff base complex alternative to traditional organic fluorophores in LSCs applications. For example, Lumogen Red, i.e., the state-of-art of fluorophores for LSCs, is nowadays quoted at about 7500 € per kg by BASF, which is an issue that definitely affects the final cost of these devices, thus limiting their worldwide distribution.
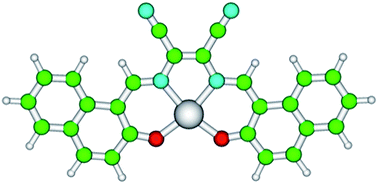 | ||
| Fig. 1 Structure of zinc N,N′-bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile (ZnL). Atoms are represented by spheres of different colours: Zn = grey, C = green, O = red, N = cyan, and H = white. | ||
Herein, a joint experimental and computational study of ZnL spectroscopic properties in different solutions is reported. By analyzing the electronic structure features and optical properties, the nature of the absorbing and emitting states was dissected, identifying the role of Zn and the importance of Zn–solvent interactions in determining the observed spectra. Motivated by these results, the optical features of ZnL were investigated when dispersed in transparent amorphous poly-methyl-methacrylate (PMMA) thin films, aiming at the realization of a new LSC device. While bulk-plate configurations ensure a larger number of fluorophores embedded without generating significant efficiency losses due to the concentration, the thin film procedure offers numerous advantages in the experimental process,40,41 such as limited use of materials, and easy and fast setup, which are the conditions required for the large-scale preparation of LSC samples.
Experimental section
Materials
Poly(methyl methacrylate) (PMMA, Aldrich, Mw = 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, acid number < 1 mg KOH per g), was used as received. N,N′-Bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile (H2L) and its neutral Zn(II) complex were prepared and characterized using a literature procedure.42 Unless stated otherwise, the commercially available materials were used as received.
000 g mol−1, acid number < 1 mg KOH per g), was used as received. N,N′-Bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile (H2L) and its neutral Zn(II) complex were prepared and characterized using a literature procedure.42 Unless stated otherwise, the commercially available materials were used as received.
Synthesis of H2L: 1 g (5.8 mmol) of 2-hydroxy-1-naphthaldehyde, 0.31 g (0.29 mmol) diaminomaleonitrile were mixed in a mixture of DMF (40 mL) and acetic acid (10 mL) and stirred at room temperature. After adding 1 drop of concentrated sulphuric acid, the mixture was stirred at 80 °C for 8 h. The deep green precipitate was filtered, washed with DMF and ethanol, and air-dried to yield 0.5 g (41.3%) of product. Mp > 300 °C. Anal. calc. for C26H16N4O2 (%): C, 74.99; H, 3.87; N, 13.45. Found: C, 75.15; H, 3.92; N, 13.38. FT-IR (KBr) (cm−1): 3421 (OH), 2218 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) 1618 (C
N) 1618 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, CDCl3): δ = 7.21 (d, 2H; ArH), 7.34 (dd, 2H; ArH), 7.50 (dd, 2H, ArH), 7.80 (d, 2H, ArH), 7.84 (d, 2H, ArH), 8.00 (d, 2H, ArH), 9.70 (s, 2H; CHN), 12.93 (s, 2H; OH).
N). 1H NMR (300 MHz, CDCl3): δ = 7.21 (d, 2H; ArH), 7.34 (dd, 2H; ArH), 7.50 (dd, 2H, ArH), 7.80 (d, 2H, ArH), 7.84 (d, 2H, ArH), 8.00 (d, 2H, ArH), 9.70 (s, 2H; CHN), 12.93 (s, 2H; OH).
Synthesis of ZnL: a mixture of 1 g (5.8 mmol) of 2-hydroxy-1-naphthaldehyde, 0.31 g (0.29 mmol) diaminomaleonitrile and 0.64 g (0.29 mmol) of Zn(CH3COO)2·2H2O were mixed in DMF (100 mL) and stirred at room temperature for 1 h. The resulting mixture was filtered and cooled in a freezer, which provided the appearance of black crystals (60% yield). Anal. calc. for C32H28N6O4Zn ([ZnL]·2DMF) (%): C, 61.40; H, 4.51; N, 13.42. Found: C, 61.62; H, 4.40; N, 13.54. FT-IR (KBr) (cm−1): 2213 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) 1615 (C
N) 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, CDCl3): δ = 6.73 (d, 2H; ArH), 7.00 (m, 2H; ArH), 7.11 (m, 2H, ArH), 7.36 (d, 2H, ArH), 7.51 (d, 2H, ArH), 7.60 (d, 2H, ArH), 9.31 (s, 2H; CHN).
N). 1H NMR (300 MHz, CDCl3): δ = 6.73 (d, 2H; ArH), 7.00 (m, 2H; ArH), 7.11 (m, 2H, ArH), 7.36 (d, 2H, ArH), 7.51 (d, 2H, ArH), 7.60 (d, 2H, ArH), 9.31 (s, 2H; CHN).
Preparation of ZnL/PMMA films
Different ZnL/PMMA thin films were prepared by drop casting, i.e., pouring a 0.8 mL chloroform solution containing 30 mg of the polymer and the proper amount of dye to obtain concentrations in the range of 0.05–2.2 wt% over a 35 × 50 mm area of a glass surface. The glass slides were cleaned with chloroform and immersed in 6 M HCl for at least 12 h. They were then rinsed with water, acetone and isopropanol and dried for 8 h at 120 °C. Solvent evaporation was performed on a warm hot plate (about 30 °C) and in a closed environment. The film thickness was measured using a Starrett micrometer to be 25 ± 5 μm. The PMMA films were removed easily with a spatula after immersion in water so that they can be stored for successive measurements and comparison by attaching them to a 50 × 50 × 3 mm optically pure glass substrate (Edmund Optics Ltd BOROFLOAT window 50 × 50 TS) with a high-purity silicone oil with a refractive index comparable to PMMA and glass (i.e., poly(methylphenyl siloxane), 710 fluid, Aldrich, refractive index n = 1.5365). The absorption and emission properties of such devices showed negligible differences with the freshly prepared ones.Characterizations
The melting points were recorded on a hot-stage microscope (Reichert Thermovar). The FT-IR spectra were obtained with the help of a Perkin Elmer Spectrum One spectrometer in KBr dispersions. The NMR spectra were obtained at room temperature at 300 MHz (1H) and were referenced to TMS or to the residual protons of deuterated solvents. The absorption spectra were obtained at room temperature on a Perkin-Elmer Lambda 650 spectrometer. The fluorescence spectra were obtained at room temperature on a Horiba Jobin-Yvon Fluorolog®-3 spectrofluorometer equipped with a 450 W xenon arc lamp, double-grating excitation and single-grating emission monochromators. The emission quantum yields of the solid samples were obtained using a 152 mm diameter “Quanta-phi” integrating sphere coated with Spectralon® and mounted in the optical path of the spectrofluorometer, using a 450 W xenon lamp as an excitation source coupled with a double-grating monochromator for selecting the wavelengths.Photocurrent measurements43
A proper apparatus was constructed and composed of plywood wooden box 15 × 15 × 30 cm with walls 1.5 cm thick. A removable cover hosting housing for a solar lamp is present at the top. During the measurement, a solar lamp TRUE-LIGHT® ESl E27 20 W was used. Two 50 × 3 mm slits were carved out at 5 cm from the bottom of the box to exactly fit the LSC systems (dimensions 40 × 50 × 3 mm) so that the minimum amount of light would come out during the measurement conditions. On the outer side of the slit, a set of three 1 × 1 cm photodiodes (THORLABS FDS1010 Si photodiode, with an active area of 9.7 × 9.7 mm and high responsivity (A W−1) in the spectral range of 400–1100 nm (Fig. S1†)) connected in parallel was placed and coupled to a multimeter (KEITHLEY Mod. 2700) for photocurrent measuring.Efficiency measurement using a PV-cell43
A different set of LSC samples was prepared to measure the concentration efficiency by attaching a Si-PV cell (IXYS SLMD121H08L mono solar cell 86 × 14 mm, with a solar cell efficiency of 14% and a fill factor > 70%) to one edge of the sample. This set of samples was made covering the 40 × 50 area of the previously introduced optically pure glass slabs with a 25 ± 5 μm ZnL/PMMA thick film. One edge of the LSC was connected to a Si-based PV cell masked to cover just the LSC edge (50 × 3 mm) using silicone grease, whereas the remaining edges were covered with aluminum tape. These devices where then placed over a white poly(ethylene terephthalate) scattering sheet (Microcellular® MCPET reflective sheet, ERGA TAPES Srl) and placed about 20 cm under a solar lamp (TRUELIGHT® ESL E27 20 W, with a correlated colour temperature of 5500 K). The efficiency was reported to be ηopt, which is the ratio between the short circuit current of the PV cell attached the LSC edges under illumination of a light source (ILSC) and the short circuit current of the bare cell placed perpendicular to the light source (ISC).Computational details
The ZnL molecular structure, vertical excitation and emission properties were characterized by quantum chemical calculations based on density functional theory (DFT) and time-dependent DFT (TD-DFT) approaches.44–47 Several tests were performed to choose the most accurate level of theory at the most feasible computational costs. After comparing the different density functional models, the most commonly employed B3LYP hybrid DFT functional was chosen.48,49 The use of a larger content of non-local exact exchange or a long-range corrected hybrid-DFT approach did not provide significant improvements over B3LYP (see ESI, Table S1†). The calculations address the prediction of intra-ligand electronic transitions. For this process, the choice of the basis set for the ligand atoms is crucial. The double- and triple-ζ basis set from the Pople's and Dunning's series was tested, plus the addition of diffuse and polarization functions. After the convergence tests (see ESI, Table S2†), we adopted the 6-311++G(d,p)50,51 basis set for C, N, O and H atoms. For the Zn atom, the LANL2TZ+ effective core potential (ECP) and basis set were used.52,53 Testing other different ECP-basis set combinations for Zn did not affect the predicted ZnL electronic structure. In structural optimizations, molecular frequencies and electronic transition calculations, the bulk solvent effects have been taken into account using the well-known Polarizable Continuum Model (PCM) implicit solvation scheme.54–56 In particular, the ZnL adsorption and emission properties in solution have been calculated according to the state-specific PCM approach.57,58 All the calculations were performed with the Gaussian 09 suite of programs for quantum chemistry.59Results and discussion
Optical characterization of the ZnL in solution
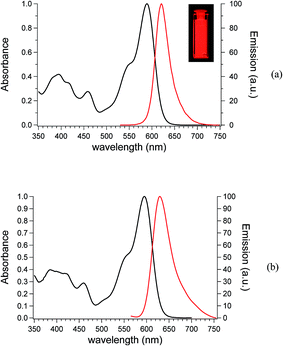
ZnL was prepared according to Liuzzo and Di Bella:42,60 the FTIR spectra showed the stretching of imines groups (1615 cm−1) and nitrile groups (2213 cm−1) of ZnL, whereas 1H NMR and elemental analysis confirmed the ZnL composition.The UV-Vis absorption spectra of dilute dioxane (DOX) and tetrahydrofuran (THF) solutions of ZnL are shown in Fig. 2. The absorption spectra indicate that the electronic transition should be attributed to intramolecular charge transfer (ICT) due to the conjugated nature of the Schiff base complex, as observed in other salicylaldiminate systems containing the diaminomaleonitrile bridge.60,61 The absorption maxima of ZnL in dioxane (dielectric constant = 2.25, Fig. 2a) and THF (dielectric constant = 7.58, Fig. 2b) are located at 589 nm and 595 nm, respectively. The emission spectra (λexc. = 450 nm) of ZnL in dioxane and THF solutions exhibit intense unstructured bands with maxima at 621 nm (Stokes shift = 32 nm) and 630 nm (Stokes shift = 35 nm), respectively.
The electronic features behind these ZnL optical spectra were investigated by state-of-the-art Density Functional Theory (DFT) and Time Dependent-DFT (TD-DFT) calculations. Structural optimizations of ground- and first-excited electronic states were performed with the polarizable continuum model (PCM) for DOX and THF solutions. In addition, a discrete-continuum cluster model approach was applied with explicit solvent molecules and PCM to account for both short-range and long-range solute–solvent interactions.62,63 In these cases, two solvent molecules have been placed in perpendicular direction to the ZnL plane above and below the planar dye, with oxygen atoms pointing toward the Zn cation. The computed structural data are reported in ESI (Tables S3 and S4†). The predicted adsorption and emission vertical transition energies are reported in Fig. 3 together with the ZnL molecular orbital energy levels in dioxane and tetrahydrofuran, and the isodensity surface plots for the molecular orbitals that are involved in these transitions (HOMO–LUMO).
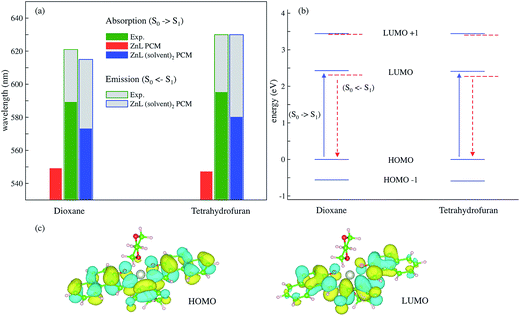 | ||
| Fig. 3 (a) TD-DFT vertical excitation and emission energies computed on the ground (S0) and excited state (S1) minima, respectively, in dioxane and THF solutions. Implicit PCM and discrete-continuum, (solvent) 2-PCM solvation models are compared on absorption energies. The experimental values are from Fig. 2; the grey area represents the Stokes shift. (b) Molecular orbital (MO) energy levels for ZnL(dioxane)2–PCM and ZnL(tetrahydrofuran)2–PCM: blue solid lines represent the MOs at the ground state minimum, whereas the dashed red lines represent the MO levels at the S1 TD-DFT optimized structure; the vertical transitions of interest in absorption (solid blue arrow) and emission (dashed red arrow) involve only HOMO and LUMO orbitals. (c) Iso-density surface plots of HOMO and LUMO molecular orbitals for the ZnL(dioxane)2–PCM system (positive and negative values are depicted in yellow and cyan, respectively, with a contour threshold value of 0.02). | ||
From a computational perspective, the implicit solvation scheme was found to be insufficient to predict the solvent effects: the vertical absorption values are far off the experimental ones for both DOX and THF. Moreover, the PCM is not even able to distinguish between the two solvents. When explicit molecules are included, the computed absolute excitation values for ZnL–DOX2–PCM and ZnL–THF2–PCM still present an error when compared to experiments (∼2%), but it falls within the expected accuracy of this approach. Moreover, the discrete-continuum predictions are much closer than the PCM ones to the reference values and, noteworthy, they have also been able to describe the red shift of ZnL absorption in THF with respect to the DOX solution. The ZnL–DOX2–PCM and ZnL–THF2–PCM model systems were also employed to predict the excited-state minimum energy structures and the corresponding vertical emission energies. The predicted emission energies are in better agreement with experiments than the adsorption values. The electronic excitation process has an intra-molecular charge-transfer (CT) nature. Fig. 3c shows the HOMO and LUMO molecular orbitals that are involved in the predicted and observed transitions. The HOMO is well localized across the aromatic moieties, whereas the LUMO has an important localization on the CN moieties. These qualitative features are the same in both DOX and THF solutions. This behaviour is in line with the fluorescence of zinc(II) complexes, which are determined only by the π–π* transition of the organic ligand because the d shell of the central ion is filled completely.64 Moreover, MO analysis also explains why the absorption and emission maxima of ZnL are red-shifted in THF because of its higher dielectric constant than dioxane, THF provides a small but sensible stabilization of the CT excited state.
Despite the slight overestimation of the Stokes shift, the proposed discrete-continuum model provided overall a reliable qualitative estimate of the electron absorption and emission transitions. Therefore, the same protocol to model the electronic structure of ZnL was applied when embedded into the PMMA thin film, the target LSC host matrix. PMMA is an amorphous polymer matrix with a dielectric constant of 2.6–2.8. Therefore, to model the polymer matrix, the PCM model was safely employed (setting the dielectric constant to the average value of 2.7). Moreover, the presence of exposed oxygen atoms in the PMMA lateral residues can lead to the direct coordination of these oxygen moieties to the Zn ion in ZnL. Thus, for modelling the ZnL/PMMA system a discrete-continuum approach was used, as carried out with DOX and THF. Table S5 in ESI† lists the main structural and electronic features of the ground and excited state minima. The TD-DFT predicted absorption and emission energies (λabs = 577 nm; λems = 619 nm) are very close to the DOX ones, with a similarly convenient Stokes shift (∼42 nm). Such behaviour was expected because the chemical nature of PMMA lateral residues and PMMA bulk dielectric constant are similar to those of the DOX solution. Motivated by these positive ab initio results on the structural properties and electronic transition energies, the experimental characterization of ZnL/PMMA films for LSC applications was performed.
Optical characterization of the ZnL/PMMA films
Owing to the aforementioned opto-electronic properties, ZnL was also investigated when dispersed in the transparent and totally amorphous polymer matrix of PMMA. PMMA was selected as the polymer matrix because of its completely amorphous state, which confers the material optical transparency and good mechanical properties. PMMA is also cheap and commercially available, characteristics that make this polymer a perfect candidate for large scale LSC applications.65,66The optical characteristics of ZnL in the PMMA matrix are shown in Fig. 4 with the maximum absorption and emission bands found at 590 nm and 624 nm, respectively, with a Stokes shift of 34 nm. ZnL absorbs light down to 500 nm, due to a second structure-less band centred at 450 nm. Both the emission maximum and Stokes shift in PMMA are comparable to those recorded in dioxane solutions due to the similar dielectric constants.
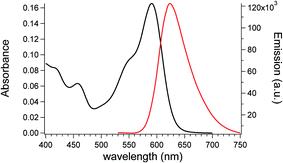 | ||
| Fig. 4 Absorption and emission spectra (λexc. = 450 nm) of a 0.3 wt% ZnL/PMMA film with a thickness of 25 ± 5 μm. | ||
The absolute fluorescent quantum yield (QY) of ZnL in PMMA reached an average value of 23%, which was maintained even at the highest concentration investigated (i.e. 22.5% for 1 wt% ZnL/PMMA film). This value is lower than that of the DOX solution (39%) but still comparable to that recorded for fluorophores dispersions at such a long wavelength emission (∼630 nm).67
Measurements of the LSC efficiencies are usually performed by attaching PV modules to the concentrating system and irradiating it with a light source that emulates the solar conditions.25,26,40 While this approach is effective for evaluating the ultimate LSC performance, it has created a lot of confusion in the literature data,14 because many research groups make use of different and not always directly comparable conditions and experimental setups on their pursue to the best performing LSC system. Moreover, sometimes LSCs are evaluated with parameters referred to other solar generating systems, such as photon-per-electron efficiencies or fill factors, which are meaningless in this specific case because the LSC itself is not an energy generating device but achieves light concentration only.14 To assess the performances as LSC, an optically pure 50 × 50 × 3 mm glass was coated with ZnL/PMMA films with a thickness of 25 ± 5 μm. Photocurrent measurements were accomplished using a home-built apparatus43 (see Experimental part) using a set of three 1 × 1 cm photodiodes assembled in a parallel fashion. Photodiodes are ideal for measuring light sources in the LSC emission range by converting the optical power to an electrical current, allowing for a fast, precise and reproducible response, even with different sets of samples. This approach was used to study the best working conditions for different dye/polymer LSC systems because the response curves of the photodiodes and the utilized PV module do not differ significantly.
The photocurrents measured for a set of samples based on ZnL/PMMA thin films are reported in Fig. 5.
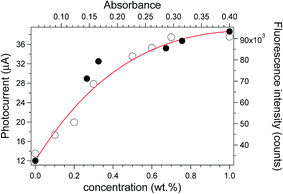 | ||
| Fig. 5 Fluorescence peak emission intensity vs. absorbance (black filled circles) of ZnL/PMMA films with a thickness of 25 ± 5 μm with increasing dye concentration and photocurrent (open circles) measured for the same films at different dye contents (wt%). The photocurrents were fitted with eqn (1) (red curve) with the parameters listed in Table 1 (see below). | ||
The data followed a peculiar trend, i.e. photocurrent increasing with ZnL content and levelling off at the highest concentration investigated. This trend is in accordance with the plot of the fluorescence emission intensity vs. absorbance, which was reported to reveal possible loss of the absorbed photons via non-radiative pathways.19 In detail, the emission intensity was found to increase linearly with the absorbance of ZnL/PMMA up to 0.3 wt% of fluorophore, indicating a negligible effect of dissipation phenomena. Conversely, when the concentration was increased further a deviation from linearity is observed, suggesting that dissipative phenomena occur.
It can be noted that the photocurrent behaviour fits quite well with eqn (1):
| ηopt = ε′ce−μoptc + D | (1) |
ε′ ∝ he−![[l with combining macron]](https://www.rsc.org/images/entities/i_char_006c_0304.gif)
| (2) |
μopt ∝ μ′′(QY,p)![[l with combining macron]](https://www.rsc.org/images/entities/i_char_006c_0304.gif)
| (3) |
![[l with combining macron]](https://www.rsc.org/images/entities/i_char_006c_0304.gif) is the mean path length of the radiation in the optical system and μ′′ is a term depending on both QY and the probability of fluorescence re-absorption (p), being greater at high p and low QY. D is an empirical constant added because even an empty system of transparent material (c = 0) is capable of trapping some light through surface and bulk defects due to a scattering phenomenon.
is the mean path length of the radiation in the optical system and μ′′ is a term depending on both QY and the probability of fluorescence re-absorption (p), being greater at high p and low QY. D is an empirical constant added because even an empty system of transparent material (c = 0) is capable of trapping some light through surface and bulk defects due to a scattering phenomenon.
Eqn (1) was recently determined inspired by the study of Goezberger27 who proposed in 1977 an effective method to evaluate the LSC efficiency. Both ε′ and μopt must be considered to be completely empirical because even the most accurate estimations require strong approximations. Nevertheless, the determination of how they affect the final ηopt is straightforward for determining the LSC performance. It can be noted that ε′ is a coefficient related to the absorption properties of the dye/polymer system, whereas μopt combines all the fluorescence quenching mechanisms due to the dye. Therefore, an optimal dye/polymer system should present a high ε′ and a small μopt so that the maximum efficiency is shifted to higher concentrations and the curve rises steadily under the influence of the linear part (eqn (1)). A complete and exhaustive determination of eqn (1) was recently reported by our group.43
The fitting parameters, reported in Table 1, were compared with those recently gathered for PMMA films with the same thickness of 25 ± 5 μm but containing Lumogen Red F350 (LR),43 as reference because it is considered the state-of-the-art in dyes for LSC applications.14
| Entry | ε′ | μopt | D |
|---|---|---|---|
| ZnL/PMMA | 65 | 0.90 | 12 |
| LR/PMMA | 140 | 0.45 | 20 |
The fitting parameters of ZnL/PMMA films were different from those of LR/PMMA: lower values for ε′ and slightly higher μopt were collected. On the contrary, the D values were similar for all the dye/PMMA systems, suggesting that the contribution of non-fluorescent trapping is more or less the same for samples with the same thickness. The smaller ε′ is a result of the lesser extinction coefficient of ZnL compared to that of LR in PMMA, ε′ being related to the light absorption properties of the system (Fig. S2†).
Nevertheless, ZnL showed a μopt value comparable to that of LR, possibly due to the larger Stokes shift (34 nm for ZnL against 23 for LR43) and a more red-shifted emission (624 nm for ZnL against 609 for LR43), notwithstanding the lower QY compared to that of LR.43 Attempts to evaluate the effect of self-absorption on the LSC optical performances have been thoroughly reviewed in the literature.40,68–71 Re-absorption of emitted photons by subsequent dye molecules via overlap of emission and absorption bands appears to be the limiting factor in respect to the efficiency of the concentrator.14,72 Moreover, a more red-shifted emission agrees better with the typical responsivity curve of photodiodes (Fig. S1†).
The ZnL/PMMA films with the highest photocurrent, i.e. those containing 0.7 and 1 wt% of ZnL, were analysed using a Si-based PV cell attached to one edge of the concentrator, as described in the Experimental section. The optical efficiency, ηopt (Table 2), was evaluated from the concentration factor C, which is the ratio between the short circuit current measured in the case of the cell over the LSC edge (ILSC) and short circuit current of the bare cell when perpendicular to the light source (ISC) (eqn (4)):
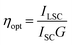 | (4) |
| Entry | wt% | C | ηopt (%) |
|---|---|---|---|
| ZnL/PMMA | 0.7 | 0.92 | 6.92 |
| 1.0 | 0.90 | 6.76 | |
| LR/PMMA | 0.7–1.0 | 0.94–1.06 | 7.0–8.0 |
The calculated C and ηopt for the ZnL/PMMA system with the highest photocurrents were comparable to those gathered from the LSC based on LR in the same range of fluorophore concentration and geometrical factor. This suggests that despite the 23% of QY in PMMA, the red-emitting ZnL fluorophore yields LSC system with noteworthy optical efficiencies, possibly due to the larger Stokes shift and emission in the range of the highest quantum efficiency of the PV cell (600–770 nm, Fig. S5†).14,73
Preliminarily experiments aimed at the photostability determination of ZnL/PMMA thin films, revealed that the systems lost only 2–3% of their emission during the first 15 min of continuous light irradiation at 450 nm (i.e., at λexc.) with a 450 W Xe arc lamp under aerobic conditions. Moreover, the expected temperatures reached under continuous solar irradiation would be less than 40–50 °C,74 which is well below the degradation temperatures of the prepared materials42 and devices.
Conclusions
We have demonstrated that ZnL, a highly emissive red-emitting zinc(II) complex of the donor–acceptor–donor (D–A–D) type ligand N,N′-bis(2-hydroxy-1-naphthylidene)-diaminomaleonitrile, once embedded into PMMA, improves the optical efficiencies of the thin films, which make them suitable for the preparation of LSCs. ZnL displayed emission bands with maximum at λ > 620 nm and a Stokes shift > 30 nm, both in solutions and in PMMA, whose QY reached values of about 23%. From the computational perspective, a discrete-continuum model approach was tested and validated to predict the adsorption and emission properties of ZnL in DOX and THF solutions by DFT and TD-DFT calculations. Despite the simplicity of the model, the results provided a reliable qualitative description of the structural features and transition energy trends in the different solvents. The same model when applied to describe the ZnL in PMMA film provided results that were confirmed by experiments. In light of these peculiar features, the ZnL/PMMA system yields C and ηopt of maximum 0.92 and 6.92, respectively, which were comparable to that evaluated from LSC based on LR in the same range of fluorophore concentration and geometrical factor. These performances were attributed to the larger Stokes shift of ZnL that prevents the loss of efficiencies due to self-absorption and moves ZnL emission more within the range of the highest quantum efficiency of the PV cell. Future approaches for ηopt enhancement should adopt new synthetic strategies aimed at increasing the fluorophore QY while maintaining the emission maxima >600 nm. Considering the easy and economic preparation, all findings consistently support the effective use of the red-emitting zinc complex in the realization of cost-effective LSC.Acknowledgements
The study leading to these results has received funding from MIUR-FIRB (RBFR122HFZ) and in part from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. [320951]. ERGA TAPES Srl and BASF Italia S.p.A are kindly acknowledged for providing some free samples of “Microcellular® MCPET reflective sheet” and Lumogen Red F350, respectively. Dr Marco Carlotti is kindly acknowledged for helpful discussions.References
- M. Debije, Nature, 2015, 519, 298–299 CrossRef CAS PubMed.
- J. L. Sawin, Renewables Global Status Report, Renewable Energy Policy Network for the 21th Century, Paris, 2014 Search PubMed.
- W. G. J. H. M. van Sark, Renewable Energy, 2013, 49, 207–210 CrossRef CAS.
- W. T. Welford and R. Winston, Optics of nonimaging concentrators, Light and solar energy, 1978 Search PubMed.
- A. Rabl, Sol. Energy, 1976, 18, 93–111 CrossRef.
- A. Gombert, J. C. Miñano, P. Benitez and T. Hornung, in Photon Management in Solar Cells, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, pp. 153–182, DOI:10.1002/9783527665662.ch6.
- R. M. Swanson, Prog. Photovoltaics, 2000, 8, 93–111 CAS.
- G. Smestad, H. Ries, R. Winston and E. Yablonovitch, Sol. Energy Mater., 1990, 21, 99–111 CrossRef CAS.
- G. A. Madhugiri and S. R. Karale, International Journal of Modern Engineering Research, 2012, 2, 1381–1385 Search PubMed.
- N. Tiwari and K. Mishra, Advanced Renewable Energy Sources, Royal Society of Chemistry, London, 2012 Search PubMed.
- J. C. Goldschmidt, L. Prönneke, A. Büchtemann, J. Gutmann, L. Steidl, M. Dyrba, M.-C. Wiegand, B. Ahrens, A. Wedel, S. Schweizer, B. Bläsi, R. Zentel and U. Rau, in Photon Management in Solar Cells, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, pp. 283–321, DOI:10.1002/9783527665662.ch11.
- S. T. Bailey, G. E. Lokey, M. S. Hanes, J. D. M. Shearer, J. B. McLafferty, G. T. Beaumont, T. T. Baseler, J. M. Layhue, D. R. Broussard, Y.-Z. Zhang and B. P. Wittmershaus, Sol. Energy Mater. Sol. Cells, 2007, 91, 67–75 CrossRef CAS.
- A. Sanguineti, M. Sassi, R. Turrisi, R. Ruffo, G. Vaccaro, F. Meinardi and L. Beverina, Chem. Commun., 2013, 49, 1618–1620 RSC.
- M. G. Debije and P. P. C. Verbunt, Adv. Energy Mater., 2012, 2, 12–35 CrossRef CAS.
- M. Tonezzer, D. Gutierrez and D. Vincenzi, Sol. Cell Nanotechnol., 2014, 293–315, DOI:10.1002/9781118845721.ch12.
- Y. S. Lim, S. Y. Kee and C. K. Lo, Sol. Cell Nanotechnol., 2014, 271–291, DOI:10.1002/9781118845721.ch11.
- R. Turrisi, A. Sanguineti, M. Sassi, B. Savoie, A. Takai, G. E. Patriarca, M. M. Salamone, R. Ruffo, G. Vaccaro, F. Meinardi, T. J. Marks, A. Facchetti and L. Beverina, J. Mater. Chem. A, 2015, 3, 8045–8054 CAS.
- F. Meinardi, A. Colombo, K. A. Velizhanin, R. Simonutti, M. Lorenzon, L. Beverina, R. Viswanatha, V. I. Klimov and S. Brovelli, Nat. Photonics, 2014, 8, 392–399 CrossRef CAS.
- G. Griffini, M. Levi and S. Turri, Renewable Energy, 2015, 78, 288–294 CrossRef CAS.
- Y. Zhao, G. A. Meek, B. G. Levine and R. R. Lunt, Adv. Opt. Mater., 2014, 2, 606–611 CrossRef CAS.
- W. E. Benjamin, D. R. Veit, M. J. Perkins, E. Bain, K. Scharnhorst, S. McDowall, D. L. Patrick and J. D. Gilbertson, Chem. Mater., 2014, 26, 1291–1293 CrossRef CAS.
- M. J. Currie, J. K. Mapel, T. D. Heidel, S. Goffri and M. A. Baldo, Science, 2008, 321, 226–228 CrossRef CAS PubMed.
- S. F. Daorta, M. Liscidini, L. C. Andreani, P. Scudo and R. Fusco, presented in part at the 26th European Photovoltaic Solar Energy Conference and Exhibition, Hamburg, 2011 Search PubMed.
- L. Desmet, A. J. M. Ras, D. K. G. de Boer and M. G. Debije, Opt. Lett., 2012, 37, 3087–3089 CrossRef CAS PubMed.
- J. C. Goldschmidt, M. Peters, A. Bosch, H. Helmers, F. Dimroth, S. W. Glunz and G. Willeke, Sol. Energy Mater. Sol. Cells, 2009, 93, 176–182 CrossRef CAS.
- L. H. Slooff, E. E. Bende, A. R. Burgers, T. Budel, M. Pravettoni, R. P. Kenny, E. D. Dunlop and A. Büchtemann, Phys. Status Solidi RRL, 2008, 2, 257–259 CrossRef CAS.
- A. Goetzberger and W. Greube, Appl. Phys., 1977, 14, 123–139 CAS.
- S. Flores Daorta, A. Proto, R. Fusco, L. Claudio Andreani and M. Liscidini, Appl. Phys. Lett., 2014, 104, 153901 CrossRef.
- S. M. El-Bashir, F. M. Barakat and M. S. AlSalhi, Renewable Energy, 2014, 63, 642–649 CrossRef CAS.
- I. Coropceanu and M. G. Bawendi, Nano Lett., 2014, 14, 4097–4101 CrossRef CAS PubMed.
- F. Purcell-Milton and Y. K. Gun'ko, J. Mater. Chem., 2012, 22, 16687–16697 RSC.
- S. J. Gallagher, B. C. Rowan, J. Doran and B. Norton, Sol. Energy, 2007, 81, 540–547 CrossRef.
- P. Wang, Z. Hong, Z. Xie, S. Tong, O. Wong, C.-S. Lee, N. Wong, L. Hung and S. Lee, Chem. Commun., 2003, 1664–1665, 10.1039/b303591c.
- F. D. R. Averseng, P. G. Lacroix, I. Malfant, N. Périssé, C. Lepetit and K. Nakatani, Inorg. Chem., 2001, 40, 3797–3804 CrossRef CAS PubMed.
- S. Di Bella, N. Leonardi, G. Consiglio, S. Sortino and I. Fragalà, Eur. J. Inorg. Chem., 2004, 2004, 4561–4565 CrossRef.
- H. Zhu, J. Fan, B. Wang and X. Peng, Chem. Soc. Rev., 2015, 44, 4337–4366 RSC.
- S. Enthaler and X.-F. Wu, in Zinc Catalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, DOI:10.1002/9783527675944.ch1, pp. 1–4.
- C. M. da Silva, D. L. da Silva, L. V. Modolo, R. B. Alves, M. A. de Resende, C. V. B. Martins and Â. de Fátima, J. Adv. Res., 2011, 2, 1–8 CrossRef.
- W. Qin, S. Long, M. Panunzio and S. Biondi, Molecules, 2013, 18, 12264 CrossRef CAS PubMed.
- T. Dienel, C. Bauer, I. Dolamic and D. Bruehwiler, Sol. Energy, 2010, 84, 1366–1369 CrossRef CAS.
- G. Griffini, L. Brambilla, M. Levi, M. Del Zoppo and S. Turri, Sol. Energy Mater. Sol. Cells, 2013, 111, 41–48 CrossRef CAS.
- V. Liuzzo, W. Oberhauser and A. Pucci, Inorg. Chem. Commun., 2010, 13, 686–688 CrossRef CAS.
- M. Carlotti, A. Panniello, E. Fanizza and A. Pucci, Sol. Energy, 2015, 119, 452–460 CrossRef CAS.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, 864–871 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- W. Kohn, A. D. Becke and R. G. Parr, J. Phys. Chem., 1996, 100, 12974–12980 CrossRef CAS.
- R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- A. D. McLean and G. S. Chandler, J. Chem. Phys., 1980, 72, 5639 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270 CrossRef CAS.
- L. E. Roy, P. J. Hay and R. L. Martin, J. Chem. Theory Comput., 2008, 4, 1029–1031 CrossRef CAS PubMed.
- S. Miertuš, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef.
- J. Tomasi, R. Cammi, B. Mennucci, C. Cappelli and S. Corni, Phys. Chem. Chem. Phys., 2002, 4, 5697–5712 RSC.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- R. Improta, V. Barone, G. Scalmani and M. J. Frisch, J. Chem. Phys., 2006, 125, 054103 CrossRef PubMed.
- R. Improta, G. Scalmani, M. J. Frisch and V. Barone, J. Chem. Phys., 2007, 127, 074504 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- P. G. Lacroix, S. Di Bella and I. Ledoux, Chem. Mater., 1996, 8, 541–545 CrossRef CAS.
- G. Consiglio, S. Failla, I. P. Oliveri, R. Purrello and S. Di Bella, Dalton Trans., 2009, 10426–10428, 10.1039/b914930a.
- R. Improta and V. Barone, Chem. Rev., 2004, 104, 1231–1254 CrossRef CAS PubMed.
- F. Aquilante, V. Barone and B. O. Roos, J. Chem. Phys., 2003, 119, 12323 CrossRef CAS.
- S. Chakraborty, C. R. Bhattacharjee, P. Mondal, S. K. Prasad and D. S. S. Rao, Dalton Trans., 2015, 44, 7477–7488 RSC.
- T. L. Richardson and E. Lokensgard, Industrial Plastics: Theory and Applications, Delmar Publishers Inc., Albany, NY, 1997 Search PubMed.
- L. H. Sperling, Introduction to Physical Polymer Science, John Wiley & Sons, Inc., Hoboken, New Jersey, 4th edn, 2006 Search PubMed.
- B. Valeur and M. N. Berberan-Santos, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, Germany, 2nd edn, 2013 Search PubMed.
- O. M. ten Kate, K. W. Kraemer and E. van der Kolk, Sol. Energy Mater. Sol. Cells, 2015, 140, 115–120 CrossRef CAS.
- S. F. H. Correia, P. P. Lima, P. S. Andre, M. R. S. Ferreira and L. A. D. Carlos, Sol. Energy Mater. Sol. Cells, 2015, 138, 51–57 CrossRef CAS.
- A. L. Rodarte, F. Cisneros, L. S. Hirst and S. Ghosh, Liq. Cryst., 2014, 41, 1442–1447 CrossRef CAS.
- B. Balaban, S. Doshay, M. Osborn, Y. Rodriguez and S. A. Carter, J. Lumin., 2014, 146, 256–262 CrossRef CAS.
- C. Tummeltshammer, A. Taylor, A. J. Kenyon and I. Papakonstantinou, Sol. Energy Mater. Sol. Cells, 2016, 144, 40–47 CrossRef CAS.
- M. Carlotti, G. Ruggeri, F. Bellina and A. Pucci, J. Lumin., 2016, 171, 215–220 CrossRef CAS.
- V. A. Rajkumar, C. Weijers and M. G. Debije, Renewable Energy, 2015, 80, 308–315 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23049g |
| This journal is © The Royal Society of Chemistry 2016 |