AFM structural characterization of drinking water biofilm under physiological conditions
Abstract
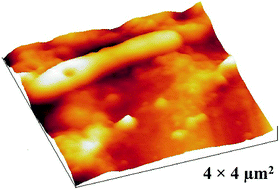
Due to the complexity of mixed culture drinking water biofilm, direct visual observation under in situ conditions has been challenging. In this study, atomic force microscopy (AFM) revealed the three dimensional morphology and arrangement of drinking water relevant biofilm in air and aqueous solution. Operating parameters were optimized to improve imaging of structural details for a mature biofilm in liquid. By using a soft cantilever (0.03 N m−1) and slow scan rate (0.5 Hz), biofilm and the structural topography of individual bacterial cells were resolved and continuously imaged in liquid without fixation of the sample, loss of spatial resolution, or sample damage. The developed methodology will allow future in situ investigations to temporally monitor structural changes in mixed culture drinking water biofilm during disinfection treatments.

 Please wait while we load your content...
Please wait while we load your content...